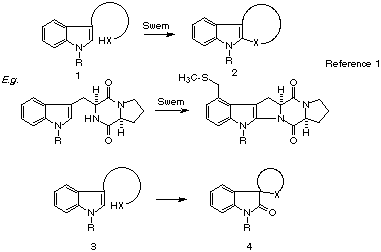
Scheme 1
Results
[A0041]
Judith Steinhoff, Pilar López-Alvarado, Sonia Miranda, Carmen Avendaño and J. Carlos Menéndez
Departamento de Química Orgánica y Farmacéutica, Facultad de Farmacia.
Universidad Complutense, 28040 Madrid, Spain.
E-mail:[email protected]
Introduction
Dimethylsulfoxide is widely employed as an oxidant, most notably in the transformation of primary alcohols into aldehydes. A recent report on the Swern oxidation of tryptamine derivatives (1) showed that the transformation of structures 1 into 2, involving overall oxidation at the C2 position of indole, could be easily achieved in moderate yields by treatment with the Swern reagent. Unexpectedly, a methylthiomethyl group was introduced at C4 of indole; this side reaction did not happen when DMSO/TFAA was employed. We now show that an alternative transformation is possible , involving the formation of a spiro ring at C3 and oxidation of the C2 position (i.e. 3 into 4) when 3-(3-hydroxyalkyl)indoles are submitted to Swern reaction conditions. This transformation takes place with preference to hydroxyl oxidation, which was not observed (Scheme 1).

Scheme 1
Results
The starting materials for our study were prepared as shown in Scheme 2. Indole derivatives 5 were formylated under Vilsmeier conditions to give compounds 6, which were submitted to Wittig reaction and then catalytic hydrogenation to afford esters 7, which were reduced to primary alcohols 8 with lithium aluminium hydride. For the preparation of secondary alcohols, Michael additions of commercially available indole derivatives 9 to several enones were carried out under ytterbium (III) catalysis (2), giving compounds 10 in quantitative yields. Sodium borohydride reduction of ketones 10 led to secondary alcohols 11.
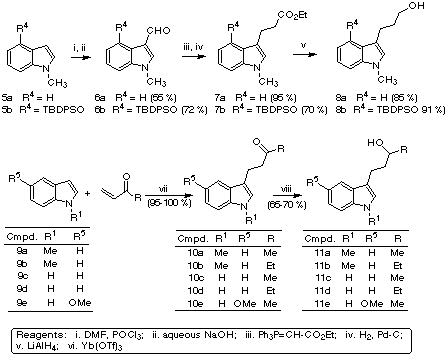
Scheme 2
When alcohols 8 and 11 were submitted to the standard Swern conditions, the results shown in Scheme 3 were obtained. Primary alcohols 8 gave spiro derivatives 12 in good or excellent yields, in a very clean reaction, and with no trace of aldehydes from oxidation of the primary hydroxyl group. The major products from the reactions starting from secondary alcohols 11 were also the spiro derivatives 13, as 2:1 mixtures of diastereomers. In some cases, compounds 14 were isolated in small yields.
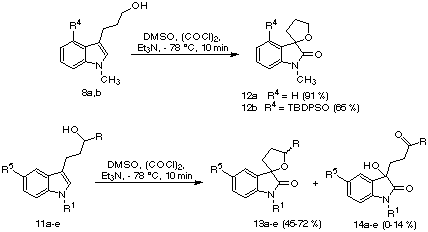
Scheme 3
The mechanism that we propose to explain the formation
of compounds 12, 13 and 14 is summarized in Scheme
4. The initial reaction between the nucleophilic C-3 position of indoles
and some of the sulfonium species present in the reaction medium leads
to intermediate I, which is then cyclized in an intramolecular nucleophilic
displacement of dimethyl sulfide to give the iminium derivative II.
This is trapped by a second molecule of dimethyl sulfoxide, giving III
and finally compounds 12 or 13 by elimination of a second
molecule of dimethylsulfide. In the cases where the cyclization is sterically
hindered (i.e., reactions starting from some of the secondary alcohols),
a part of the molecules with structure II is oxidized to IV, which
can not cyclize and reacts with water during workup to give compounds 14.
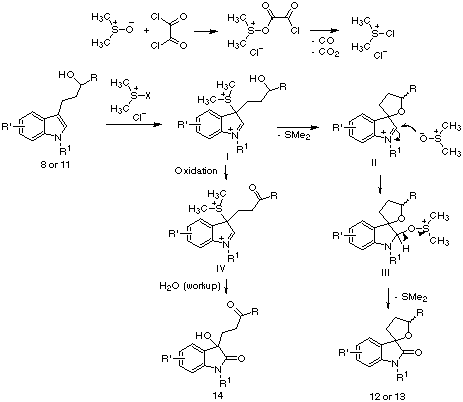
Scheme 4
In conclusion, we have found that application of
the Swern conditions to 3-(3-hydroxyalkyl)indoles leads to spiro-2-tetrahydrofuran-3-indolin-2-ones
as the main reaction products. Although the reaction is not yet
fully optimized, specially in the case of secondary alcohols, it provides
ready access to these spiro systems. Studies on the extension of this methodology
to nucleophilic groups other than hydroxyl are in progress in our laboratories.
References
(1) Bailey, P. D.; Cochrane, P. J.; Irvine,
F.; Morgan, K. M.; Pearson, D. P. J.; Veal, K. T. Tetrahedron Lett.
1999,
40,
4593.
(2) Harrington, P. E.; Kerr, M. A. Synlett1996,
1047.