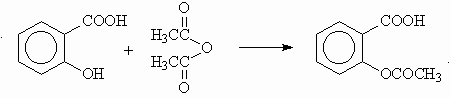
MORE Chemistry Techniques for Process Development
Ajay K. Bose,* Maghar S. Manhas, Subhendu N. Ganguly, Anju Sharma, Magaly Huarotte, Sochanchingwung Rumthao, Muthuswamy Jayaraman and Bimal K. Banik
George Barasch Bioorganic Research Laboratory, Department of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, NJ 07030, USA. *e-mail: [email protected]
Received: 18 September 2001 / Uploaded 19 September 2001
Introduction
Microwave assisted chemistry is only 15 years old. Early workers conducted chemical reactions in sealed vessels that led to occasional explosions. Microwave-induced Organic Reaction Enhancements (MORE) chemistry techniques,1,2 developed in our laboratory at Stevens Institute of Technology, are conducted in open vessels with little or no solvents. These techniques are free of the risk of explosion. MORE chemistry reactions are highly accelerated; they are cleaner than conventional reactions and lead to higher atom economy (i.e., less chemical waste).
Dipolar reactant molecules in the liquid phase absorb microwaves directly and are raised to high energy levels that may allow a reaction to take place. The energy profile in a microwave-assisted reaction may be quite different from that in a reaction mixture that is heated conventionally by conduction/convection. In a solvent-less reaction all the microwave energy is directly absorbed by the reactant molecules. Under these conditions, the "non-thermal microwave effect", if any, will be operative at high efficiency.
Recently, data have appeared in the literature purporting to show that microwaves are just a convenient way of heating and that a number of microwave reactions and conventional reactions proceed at essentially the same rate. Most of these reactions have been conducted, however, with millimolar quantities of reactants in comparatively large amounts of solvents. Therefore, if the solvent absorbs microwaves efficiently, very little microwave energy is absorbed directly by the reactants. Consequently, the reactants are in effect energized by conduction/convection as in conventionally heated reaction mixtures.
There are many as yet unknown factors that influence the course of microwave
assisted reactions. Therefore, we have been collecting extensive data on
a wide variety of synthetic processes. With a large body of reproducible
data at hand, it would be much easier to postulate the quantitative effect
of microwaves on the course of various types of reactions. It is known
that glass vessels do absorb small amounts of microwave energy. We have
therefore conducted reactions with at least several grams of reaction mixtures
to minimize the effect of thermal energy provided by the glass apparatus.
Some of our reactions have been conducted at nearly kilogram levels in
commercial microwave applicators. A few representative examples of larger
scale reactions conducted under microwave irradiation are presented below.
ASPIRIN WITHOUT HEADACHE
In our earlier publications3 we have described a simplified, rapid synthesis of aspirin from salicylic acid and a slightly more than molar equivalent of acetic anhydride in domestic microwave ovens. This synthesis was then conducted on 500-800 g scale using commercial microwave applicators: Milestone Ethos Plus and Prolabo Synthewave 1000. Aspirin of high purity was obtained in 80% yield without optimizing the reaction conditions.
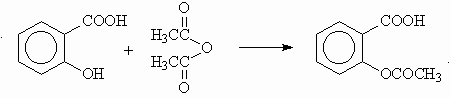
Scheme 1
To generate data for optimal reaction conditions, the acetylation reaction was conducted on a 5 g scale of salicylic acid and a calculated amount of acetic anhydride. The twelve-station assembly of the Milestone Ethos applicator was used. The irradiation time was set at 5 min. Slightly different ratios of the two reagents were used in 6 reaction vessels; one drop of phosphoric acid was used as a catalyst in one vessel while in another vessel 1% magnesium sulfate was added as an additional microwave energy absorber. The yield of aspirin in these experiments ranged between 86% and 97%; the product without recrystallization was of high purity in each case. The addition of magnesium sulfate led to excellent crystal formation of aspirin.
The Synthewave 402 applicator was used for additional experiments and it was found that when one drop of phosphoric acid was used as a catalyst, the yield was unchanged but the level of microwave energy required was reduced – presumably because of the attainment of higher efficiency of microwave energy absorption by the reaction mixture.
NOVEL N-ACETYLATION: A NEW METHOD FOR TYLENOLâ
A chance observation in our laboratory that aniline undergoes acetylation when irradiated with microwaves in dilute acetic acid solution has led to a simplified version of the Schotten-Baumann reaction. Acetaminophen (Tylenol)â can be produced in good yield in a few minutes from commercially available p-aminophenol. Thus, when 2 g of this substituted aniline was mixed with 15 mL of 80% acetic acid and exposed to low levels of microwave irradiation in a domestic microwave oven such that the bulk temperature was about 90oC, the starting material disappeared in about 4 min. A simple work up followed by one recrystallization gave 70% yield of pure acetaminophen. A parallel experiment was conducted under conventional mild reflux (at about 98oC). The progress of the reaction was monitored by thin layer chromatography. No acetylation occurred during the first 70 min. The starting material disappeared after about 2-1/2 h. The product was acetaminophen in about 60% yield after recrystallization from chloroform/hexane.
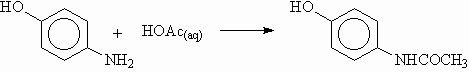
Scheme 2
A preliminary larger scale experiment with 50 g of p-aminophenol and 200 mL of 80% acetic acid was conducted in a Prolabo Synthewave 1000. Irradiation was carried out at 200W for 20 min followed by irradiation at 100W for 10 min. The final bulk temperature was about 125oC. At this point no starting material was left as shown by TLC analysis. This unoptimized run provided 55% yield of pure acetaminophen.
To test whether the "non-thermal microwave effect" observed during N-acetylation was unique for p-aminophenol, a parallel experiment was performed with p-anisidine (1g) and 80% acetic acid (20 mL). After 2 min of irradiation at 450 W in a domestic microwave oven, TLC monitoring indicated partial disappearance of the starting material. After another 2 min of irradiation at 300 W, the reaction appeared to be complete. The usual work-up gave the N-acetyl derivative in 75% yield. Acetylation was attempted under conventional heating at about 100oC. After about 20 min of heating it was found that most of the starting material was unchanged.
CLAY-CATALYZED SYNTHESIS IN AQUEOUS MEDIA: PREPARATION OF 3-CARBOXYCOUMARIN
In a recent publication Bigi et al4. have reported the optimum conditions for the preparation of 3-carboxycoumarin from salicylaldehyde and malonic acid in aqueous medium using montmorillonite KSF clay as a catalyst. After heating under reflux for 24 hours the coumarin compound was obtained in 92% yield.
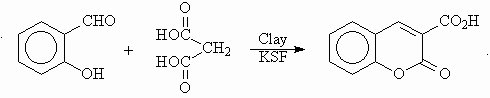
Scheme 3
We repeated this reaction and observed that no condensation took place during the first 8 h. At the end of 24 hours of heating under reflux, the yield of the coumarin compound was 89%. The identical aqueous reaction mixture was then exposed to microwaves in a domestic microwave oven for 12 min at 350 W power level. After the simple work up used by Bigi and co-workers 3-carboxycoumarin was obtained in 60% yield.
RAPID PREPARATION OF A CHIRAL b-LACTAM
In a previous publication5 we have described the microwave assisted synthesis of a chiral
b -lactam starting with an optically active
Schiff base. Condensation with benzyloxy-acetyl chloride in presence of
a tertiary base converted the Schiff base to a cis 3a
-benzyloxy-b -lactam in a few minutes under
microwave irradiation. Catalytic transfer hydrogenation with ammonium formate
and Pd/C under microwave irradiation at about 130oC led to the
corresponding optically pure a -hydroxy-b
-lactam in high yield in about 3 minutes. In the course of one day it was
thus possible to conduct a multiple step synthesis for the preparation
of 25 g of an enantiopure intermediate for the semi-synthesis of Taxol
and Taxotere.
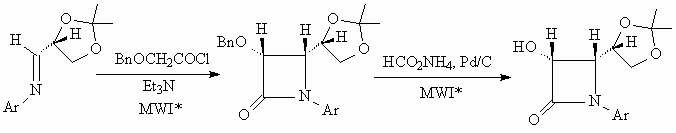
Scheme 4
A domestic microwave oven was used for this work. The scale of the reactions can be increased several folds by using the newer commercial microwave applicators one of which has been modified in our laboratory to allow reactions on a 3 kg scale.
LARGE SCALE PREPARATION OF A PROTECTED AMINO ACID AND AN a -AMINO-b -LACTAM
In an earlier publication we6 have described a rapid approach to a-amino-b -lactams using tetrachlorophthalimidoacetic acid as an intermediate. This compound was prepared on a small scale in 94% yield in 90 sec in a domestic microwave oven using a modification of a large scale phthalolylation procedure published in Organic Synthesis.7 The reaction between tetrachlorophthalic anhydride and glycine in a limited amount of N, N - dimethylformamide as the reaction medium was conducted on a molar scale to give more than 90% (about 300 g) yield of the protected amino acid after 8 min of microwave irradiation. The acid chloride derived from this intermediate reacts with Schiff bases in presence of a tertiary amine in chlorobenzene solution to give a mixture of cis and trans b-lactams. However, a high level of trans selectivity could be induced by changing the recation conditions. The solution in chlorobenzene of the Schiff base and N-methylmorpholine was first raised to a temperature of about 100oC under microwave irradiation; the acid chloride was then quickly added and the mixture was further irradiated for 3 min at low power level (300 W). The reaction product was the pure trans b-lactam.
The protective group of this trans b -lactam could be removed easily by treatment with ethylene diamine. This type of a -amino b -lactams are known intermediates for antibiotics.
MORE CHEMISTRY IN THE TEACHING LABORATORY
Several small scale (2-5g) organic and biochemical microwave assisted reactions have been organized into material for laboratories in high schools with limited facilities. 8
Experiments have also been modified to suit the needs of the undergraduate laboratories.
Apart from the shortening of reaction time, the use of MORE Chemistry in a teaching laboratory eliminates the need for heating mantles, reaction flasks and reflux condensers with ground glass joints, mechanical stirrers,etc. The smaller volume of solvent required contributes to a saving in cost and simplifies the waste disposal problem.
CONCLUDING REMARKS
There are many factors that can alter the energy profile during the course of a microwave assisted reaction. It is known that the dipole moment of a substance (or a reaction mixture) may change considerably with a change in the bulk temperature. Thus, the dipole moment of water becomes smaller at higher temperatures: therefore, as the temperature rises, water becomes a poorer absorber of microwave energy. In some reactions, the product (or intermediates or the transition states) may become strongly dipolar and thus absorb microwave energy more efficiently. In such a case, the level of microwave energy used for irradiation may be substantially reduced without slowing down the reaction. For developing an economical process, it is therefore useful to conduct a series of parallel syntheses in an appropriate commercial microwave applicator with multiple reaction vessels (for example, the Milestone Ethos Plus).
MORE chemistry techniques have been found to enhance chemical reactions in several ways. We have increasing indication that by the proper selection of the parameters of microwave irradiation, it is possible in some cases to achieve high stereoselectivity and increased atom economy.
The data collected by us and some other workers (e.g., Loupy et al9) clearly indicate that many types of reactions that require hours under conventional conditions can be conducted in minutes under microwave irradiation.
There is magic in microwaves.
ACKNOWLEDGMENTS. We wish to thank Dr. Vaidyanathan Srirajan, Dr. Ashoke
Bhattacharjee, and Dr. Yevgeniya Alkayeva and a number of participants
of our Undergraduate Projects in Technology and Medicine (UPTAM) program
for studies on small scale microwave synthesis that provided valuable background
information for our large scale microwave enhanced reactions suitable for
process development.
REFERENCES AND NOTES