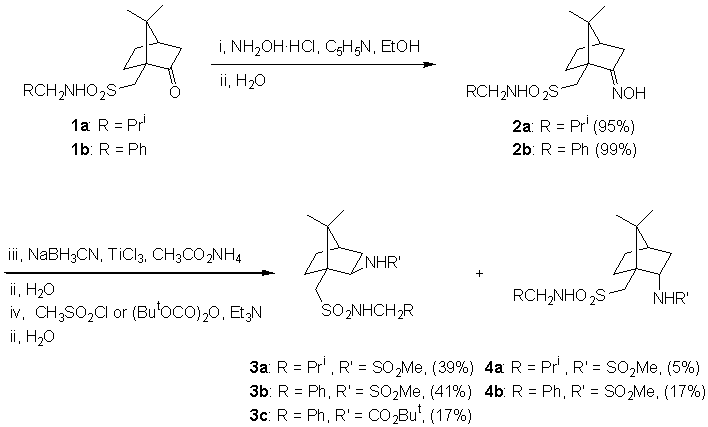
[A0008]
Disulfonamides derived from camphor: A new ligand for the titanium alkoxide-promoted addition of diethylzinc to benzaldehyde
Oscar Prieto, Diego J. Ramón and Miguel Yus*
Departamento de Química Orgánica, Universidad de Alicante, E-03080 Alicante, Spain.
Tel. +34 6 5903548, Fax +34 6 5903549, E-mail: [email protected], http://www.ua.es/dept.quimorg/
Received: 7 July 1999 / Uploaded: 17 August 1999
Abstract: The preparation of several disulfonamides 3 and 4 derived from camphor are described, as well as their use in the titanium tetraisopropoxide-promoted enantioselective addition of diethylzinc to benzaldehyde, the enantiomeric ratio being up to 82.0:18.0.
Keywords: Titanium, dialkylzinc, enantioselective addition.
Introduction
The difference in biological activity between two enantiomers, the necessity of homochiral compounds for the total synthesis of natural products and the questions of regulatory agencies about data of fate and effects of both enantiomers of a chiral pharmaceutical compound, in the course of registration, focused the interest of organic chemistry on enantioselective reactions. Among them, the nucleophilic addition to carbonyl compounds is of paramount importance in synthetic organic chemistry, as a useful tool for enantioselective carbon-carbon bond formation. Concerning this subject, the addition of diethylzinc to benzaldehyde has become a prototype in the evaluation of new chiral ligands. The use of Ti(IV) alkoxides [1] as a Lewis acid in the promotion of the above mentioned reaction has been quite popular and perhaps the most popular class of ligand has been the disulfonamide derived from chiral 1,2-diamines [2] and 1,5-diamines [3]. This fact, together with the unusual properties of hydroxycamphorsulfonamide derivatives as ligands makes these ligands able to promote the classical enantioselective addition of dialkylzinc to aldehydes in the presence of titanium(IV) alkoxide [4], as well as the new enantioselective addition of dialkylzinc to prostereogenic ketones in the presence of titanium(IV) alkoxide [5]. In this communication, we study the preparation of camphor disulfonamides derivatives and their use as ligand in the titanium-promoted addition of dialkylzinc to carbonylic compounds.
Results and Discussion
The preparation of disulfonamide ligands was performed by a three step sequence from the corresponding N-alkyl camphorsulfonamide 1; its condensation with hydroxylamine [6] gave the expected oxyme 2 with excellent yields. The reduction of oxyme using sodium cyanoborhydride, titanium trichloride and ammonium acetate [7] afforded the corresponding amine as a exo/endo mixture. The further reaction with mesyl chloride or di-tert-butyl dicarbonate gave the expected exo/endo-amide mixture, which was easily isolated by flash column chromatography (mesyl derivatives) or by crystallization (N-Boc derivative) (Scheme 1).
Scheme 1
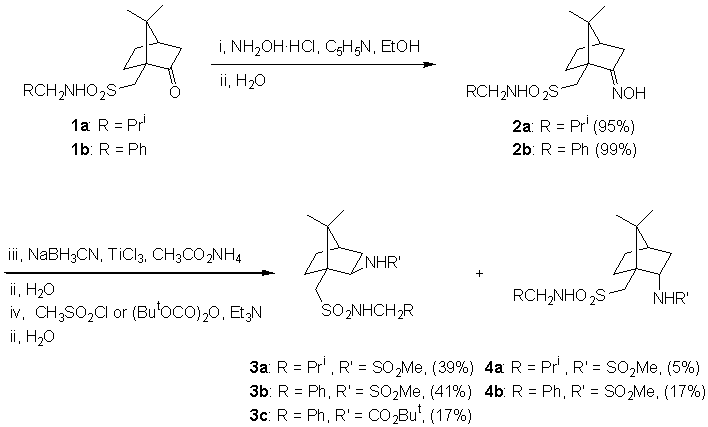
Different problems in the reproducibility and in the scale-up of the reduction made us turn to another pathway to prepare these ligands. The starting material was the Oppolzer camphorsultam (5), prepared in its turn from the corresponding camphorsulfonyl chloride according to the literature [8]. Deprotonation with butyllithium of compound 5 followed by reaction with the corresponding sulfonyl chloride gave the expected heterocyclic compound 6, which was opened by reaction with an excess of the corresponding lithium amide to yield the expected ligands 3 (Scheme 2).
Scheme 2
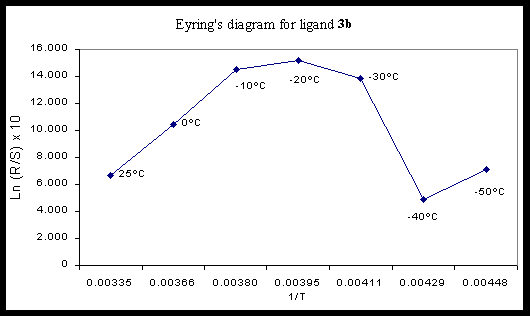
Once the ligands were obtained, they were submitted to the classical titanium tetraisopropoxide-promoted enantioselective addition of diethylzinc to benzaldehyde in toluene. The influence of temperature on the enantioselection was study for the ligand 3b. The maximum enantiomeric ratio was found at –20deg.C. Figure 1 indicates probably the existence of several reaction mechanisms in competition.
Figure 1
Other ligands were tested at –20deg.C (see Scheme 3), the following remarks being worthy to note: a) Usually, the main enantiomer in the enantioselective addition is R (in the case of using the corresponding hydroxysulfonamide the main enantiomer was S [4]); b) The role of the R group in the ligand in very important, the best e.r. being given by the aromatic one (compare ligands 3a and 3b); c) The nature of the R’ group is also important: in the case of carboxamide derivatives the secondary alcohol 7 was nearly racemic (compare ligands 3b and 3c); d) The exo/endo position of the amide group is quite important: the best result is obtained with the exo-derivative (compare ligands 3b and 4b; the same result was found in the case of hydroxysulfonamides [4]).
Scheme 3
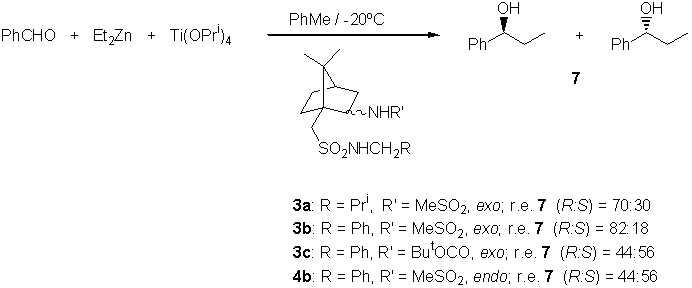
Work is in progress to develop better ligands and to improve the reaction conditions for this classical enantioselective addition, as well as to expand to the enantioselective addition of dialkylzinc reagents to prostereogenic ketones.
Conclusion
The here described disulfonamides derived from camphor are efficient ligands in the titanium alkoxide-promoted enantioselective addition of diethylzinc to benzaldehyde. The obtained results are in agreement with those obtained by us with the corresponding hydroxycamphorsulfonamide derivatives. However, the main enantiomer is the oppositein both cases in spite of the absolute configuration in the ligand stereocenters is the same. The influence of substituents in these ligands is larger than in the case of hydroxysulfonamide derivatives.
Acknowledgements
The DGICYT of the Spanish Ministerio de Educación y Cultura (no. PB97-0133) and the Generalitat Valenciana (no. DGDOC99-02-4) generously supported the work presented here.
Experimental Part
For general information, see reference [4] .
Preparation of (1S,2S,4S)-N- methanesulfonylcamphorsultam (6a). Typical Procedure.
To a solution of camphorsultam (5, 3.4g, 15.7mmol) in THF (30ml) was slowly added (ca. 10 min) a solution of n-butyllithium in hexane (12.8ml, 20.4mmol) at -78deg.C. Stirring was continued for 30 min at 0deg.C and then, the resulting solution was cooled down to -78deg.C. Methanesulfonyl chloride (1.6ml, 20.4mmol) was added to the solution and the mixture was stirred during 18 hr allowing the temperature to rise to 20deg.C. The reaction mixture was hydrolyzed with a saturated solution of ammonium chloride (15ml) and extracted with ethyl acetate (3x10ml). The organic layer was dried over anhydrous sodium sulfate and evaporated (15Torr), yielding the pure title compound (5.6g, 89%).
1
H-NMR (CDCl3): 0.97 (s, 3H), 1.23 (s, 3H), 1.30-2.05 (m, 7H), 3.18 (s, 3H), 3.45, 3.47 (2d, 2H), 3.65-3.70 (m, 1H).Preparation of (1S,2S,4S)-N-benzyl-2-methanesulfonylamino-7,7-dimethylbicyclo[2.2.1]hept-1-ylmethanesulfonamide (3b). Typical Procedure.
To a solution of benzylamine (3.0ml, 44mmol) in THF (30ml) was added a solution of n-butyllithium in hexane (19.3ml, 31mmol) at -78deg.C. After 0.5 hr a solution of compound 6a (2.82g, 7.0mmol) in THF (30ml) was added to the above solution. The resulting mixture was stirred overnigth, allowing the temperature to rise to 20deg.C. The resulting mixture was then hydrolyzed with a saturated solution of ammonium chloride (15ml) and extracted with ethyl acetate (3x10ml). The organic layer was dried over anhydrous sodium sulfate and evaporated (15Torr) to give a residue, which was purified by column chromatography (silica gel, hexane/EtOAc) affording the pure title compound (0.98g, 35%).
[a ]25D : -34.44 (c = 2, CHCl3)
IR (melted): 3297 (NH), 3031 (HC=C).
1
H-NMR (CDCl3): 0.74 (s, 3H), 0.92 (s, 3H), 1.10-1.20, 1.55-2.20 (2m, 7H), 2.71, 3.19 (2d, 2H), 3.16 (s, 3H), 3.55-3.65 (m, 1H), 4.30-4.35 (m, 2H), 5.08 (d, 1H), 5.18 (t, 1H), 7.30-7.40 (m, 5H).13
C-NMR (CDCl3): 20.15, 20.4, 27.05, 32.65, 39.2, 39.25, 44.6, 46.15, 49.5, 52.5, 59.2, 128.05, 128.25, 128.9, 136.9.MS (EI): 281 (M+-119, <1%), 139 (12), 111 (31), 101 (13), 95 (13), 86 (100), 84 (69), 83 (18), 82 (26), 58 (23), 56 (14), 54 (34), 53 (11), 44 (36), 42 (23), 41 (12).
Enantioselective addition of diethylzinc to benzaldehyde. Typical Procedure.
To a solution of ligand 3b (0.4g, 1mmol) in toluene (10ml) was added a solution of diethylzinc in toluene (6ml, 12mmol) at -20deg.C. After 10 min, titanium tetraisopropoxide (1.9ml, 6.5mmol) was added. Then, benzaldehyde (0.5ml, 5mmol) was added. The resulting solution was stirrred at the same temperature for 2 h. The reaction was then quenched with a saturated solution of ammonium chloride (10ml), filtered off through celite and extracted with ethyl acetate (3x10ml). The organic layer was dried over anhydrous sodium sulfate and evaporated (15Torr). The residue was destilled bulb to bulb to yield the alcohol 7 (0.66g 98%). The chiral ligand can be recovered by flash column chromatography.
References and Notes
[1] For a recent review on enantioselective reactions promoted by titanium(IV), see: Ramón, D. J.; Yus, M. Recent Res. Devel. Org. Chem. 1998, 2, 489-523.
[2] for examples of 1,2-cyclohexanoamide derivatives, see: a) Knochel, P. Chemtracts 1995, 205-221. b) Halm, C.; Kurth, M. J. Angew. Chem. Int. Ed. 1998, 37, 510-512. c) Fürstner, A.; Müller, T. J. Org. Chem. 1998, 63, 424-425. d) Lutz, C.; Lutz, V.; Knochel. P. Tetrahedron 1998, 54, 6385-6402. e) Lutz, C.; Graft, C.-D.; Knochel. P. Tetrahedron 1998, 54, 10317-10328. f) Pritchett, S.; Woodmansee, D. H.; Davis, T. J.; Walsh, P. J. Tetrahedron Lett. 1998, 39, 5941-5942. g) Gennari, C.; Ceccarelli, S.; Piarulli, U.; Montalbetti, C. A. G. N.; Jackson, R. F. W. J. Org. Chem. 1998, 63, 5312-5313. h) Hwang, C.-D.; Uang, B.-J. Tetrahedron:Asymmetry 1998, 9, 3979-3984. i) Takemoto, Y.; Baba, Y.; Honda, A.; Nakao, S.; Noguchi, I.; Iwata, C.; Tanaka, T.; Ibuka, T. Tetrahedron 1998, 54, 15567-15580. j) Lutz, C.; Jones, P.; Knochel. P. Synthesis 1999, 312-316. k) Arredondo, V. M.; Tian, S.; McDonald, F. E.; Marks, T. J. J. Am. Chem. Soc. 1999, 121, 3633-3639.
[3] Cernerud, M.; Skrinning, A.; Bérgčre, I.; Moberg, C. Tetrahedron: Asymmetry 1997, 8, 3437-3441.
[4] Ramón, D. J.; Yus, M. Tetrahedron: Asymmetry 1997, 8, 2479-2496.
[5] a) Ramón, D. J.; Yus, M. Tetrahedron Lett. 1998, 39, 1239-1242. b) Ramón, D. J.; Yus, M. Tetrahedron 1998, 54, 5651-5666.
[6] García Martínez, A.; Teso Vilar, E.; García Fraile, A.; de la Moya Cerero, S.; Martínez Ruiz, P. Tetrahedron: Asymmetry 1998, 9, 1737-1745.
[7] Leeds, J. P.; Kirst, H. A. Synth. Commun. 1988, 18, 777-782.
[8] Capet, M.; David, F.; Bertin, L.; Hardy, J. C. Synth. Commun. 1995, 25, 3323-3327.
Diego J. Ramón was born in Alicante (Spain) in 1965, and received his BSc (1988), MSc (1989) and PhD (1993) degrees from the University of Alicante. After spending two years as a postdoctoral fellow at the Eidgenössische Technische Hochschule in Zürich (ETH-Zentrum) he returned to the University of Alicante. In 1994, he was awarded the Prize for Young Scientists of the Spanish Royal Society of Chemistry. His current research interest is focused on organometallic chemistry and asymmetric synthesis.
Miguel Yus was born in Zaragoza (Spain) in 1947, and received his BSc (1969), MSc (1971) and PhD (1973) degrees from the University of Zaragoza. After spending two years as a postdoctoral fellow at the Max Planck Institut für Kohlenforschung in Mülheim a.d. Ruhr he returned to Spain to the University of Oviedo where he became assistant professor in 1977, being promoted to full professor in 1987 at the same university. In 1988 he moved to a chair in Organic Chemistry at the University of Alicante where he is currently the head of the Organic Chemistry Department. Professor Yus has been visiting professor at different institutions and universities such as ETH-Zentrum, Oxford, Harvard, Uppsala, Marseille and Tucson. He is co-author of about 250 papers mainly in the field of development of new methodologies involving organometallic intermediates. His current research interest is focused on the preparation of very reactive functionalized organometallic compounds and their use in synthetic organic chemistry, arene-catalyzed activation of different metals and preparation of new metal-based catalysts for homogeneous and hetereogeneous selective reactions.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0008 as the message subject of your e-mail.