[A0029]
A New Synthetic Approach toward Ring-Expanded ("Fat") Purine Nucleobases: Synthesis and Use of 5-Dichloromethyl-1-p-methoxybenzyl-4-nitroimidazole as a Versatile Intermediate
Saika Siddiqui, Bruce Gustafson, and Ramachandra, S. Hosmane*
Laboratory for Drug Design and Synthesis, Department of Chemistry &
Biochemistry
University of Maryland, Baltimore County (UMBC), 1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
Received: 17 August 1999 / Uploaded: 21 August 1999
Keywords: Synthesis, Ring-Expanded Purines, Versatile Imidazole Precursors.
INTRODUCTION
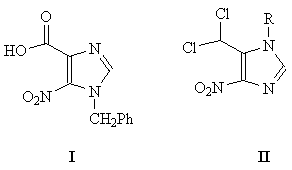
Ring expanded ("fat") purine nucleosides are of chemical, biochemical, biophysical, and medicinal interest.1-12 The majority of "fat" nucleosides reported from this laboratory in recent yeas were synthesized using the common imidazole precursor, namely 1-benzyl-5-nitro-imidazole- 4-carboxylic acid (I).5-11 However, the synthesis of I suffered from a number of drawbacks, including multi-step procedures, prolonged reaction periods (sometimes days), poor yields, tedious work-ups, and the necessity of separation of regioisomers, all of which contributed to the difficulty in preparing I on a reasonably large scale. We present here an efficient, convenient, and a versatile alternative for I in 5-dichloromethyl-1-p-methoxybenzyl -4-nitroimidazole (II). The latter has a number of novel features which will potentially not only enable the efficient resynthesis of the previously reported "fat" nucleosides from this laboratory, but will also open new ways for the synthesis of a wide variety of novel "fat" nucleosides that would otherwise be difficult to prepare using I. The novel features of II include (a) the presence of a versatile, highly reactive dichloromethyl functional group which can serve as the site of both nucleophilic and electrophilic attacks for further annulation of the appropriate side chain , (b) the potentially facile conversion of the above dichloromethyl group into a wide variety of other reactive functional groups including, but not limited to, a carboxylic acid, a carboxaldehyde, or an iminomethylene functionality, and (c) the attachment of the more conveniently removable p-methoxybenzyl protecting group at the 1-position as compared to the unsubstituted benzyl group of I. In addition, the synthesis of II is brief, convenient, reasonably good-yielding, and can be prepared on a reasonably large scale from readily available and inexpensive starting materials.

RESULTS AND DISCUSSION
The synthesis of II exploits the principle of nucleophilic substitution
on an activated heterocycle. The method is generally referred to as Vicarious Nucleophilic
Substitution or the VNS method.13 The latter is a novel way of introducing an
-functionalized alkyl chain onto activated aromatic rings.13 Although
electron-rich 5-membered nitrogen heterocycles such as imidazoles do not normally undergo
nucleophilic substitution reactions, the introduction of strong electron- withdrawing
groups such as a nitro functionality can render them vulnerable to such reactions.
The synthesis started with 4-nitroimidazole (Scheme 1), and the first step was to protect the N-H of imidazole with a benzyl group; in this case, para-methoxybenzyl chloride was employed for
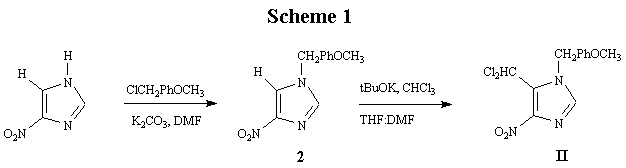
reasons described under Introduction above. Treatment of 4-nitroimidazole with para-methoxybenzyl
chloride and potassium carbonate in DMF yielded 1-p-methoxybenzyl-4-
nitroimidazole (2) as a colorless crystalline solid in 90% yield.
Compound 2 was subjected to VNS using chloroform and potassium t-butoxide
in DMF to yield the target 5-dichloromethyl-1- p-methoxybenzyl-4-nitroimidazole (II)
in 60% yield. The 1H-NMR of II in deuterated dimethyl
sulfoxide revealed the proton of 5-dichloromethyl group as a singlet at 7.96, and the
imidazole ring proton at 8.01 also as a singlet. The elemental microanalysis was
consistent and the mass spectrum showed the correct MH+ ion at m/z 316. A
mechanism for the formation of II from 2 by the VNS
method is outlined in Scheme 2. The first step, most likely the rate
determining, involves the nucleophilic attack of the carbanion formed from chloroform onto
position 5 of the imidazole ring. The subsequent base-catalyzed elimination of hydrogen
chloride, followed by reprotonation of the dichloromethylene carbon atom and the ring
aromatization produces II. The presence of a nitro group on the imidazole
ring is apparently critical for the VNS method to succeed.

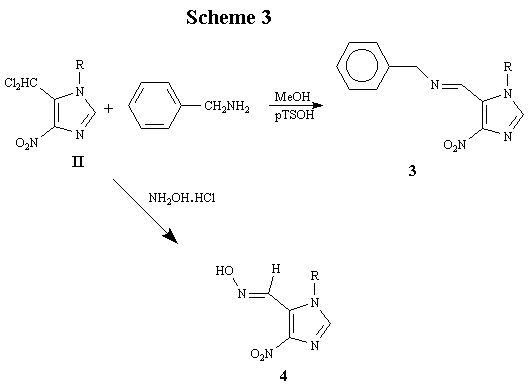
Compound II was reacted with two representative amine nuclephiles, including benzylamine and N-hydroxylamine (Scheme 3). The products 3 and 4, obtained in 44% and 63% yields, respectively, were fully characterized by spectroscopic and microanalytical data.
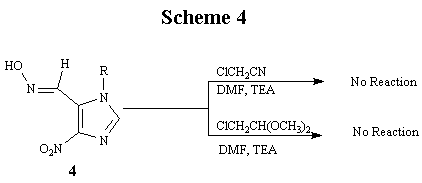
Our final goal was to demonstrate the feasibility of employing II for the synthesis of "fat" nucleosides. To this end, we decided to further explore the reactivity of 4 with alkylating agents, which, if reacted successfully, would yield product with the appropriate carbon fragment attached that would afford the 5:7 heterocyclic ring system upon ring closure. However, the reaction of 4 with either chloroacetonitrile or chloroacetaldehyde dimethyl acetal, even under forcing conditions, failed to proceed (Scheme 4). The reason for the failure is believed to be the inactivation of the oxime nitrogen atom because of conjugation with the electron-withdrawing nitro group attached at the 4-position of the imidazole ring. In order to alleviate this problem, we then decided to reduce the nitro group into an amino group before carrying out the necessary alkylation or acylation reactions.
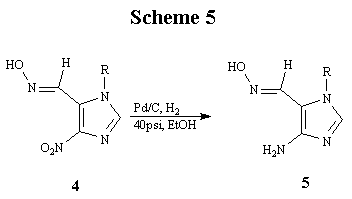
The reduction of 4 was carried out in a Parr hydrogenation apparatus, using 10% Palladium and charcoal in ethanol at 40 psi of hydrogen gas to afford the desired amine 5 in 71% yield (Scheme 5). Compound 5 was reacted with dichloroacetyl chloride to obtain 4-(2,2-dichloroacetyl)amino- 5-(N-hydroxyiminomethylene)-1-p-methoxybenzylimidazole (6) as a crystalline solid in 74%
yield. The 1H NMR of 6 exhibited the presence of D2O-exchangeable amide NH as well as the N-OH functionalities integrating for a proton each at 11.24 and 10.54 , respectively, suggesting that the reaction had taken place at the 4-amino function intead of the oxime nitrogen atom. Compound 6 was a suitable precursor for the synthesis of a representative "fat" nucleobase Indeed, the ring-closure of 6 with sodium methoxide in methanol produced the novel, 5:7-fused heterocycle III, albeit in somewhat low (34%) yield. The reason for the relatively poor yield of this reaction is not yet clear, although optimum reaction conditions for this last synthetic step are yet to be worked out. It is also not obvious as to when the methanolysis of the halide group(s) takes place, whether before or after the ring-closure. In any case, compound III, with its interesting N-oxide structure, bears a broad scope for further chemical, biochemical, biological, as well as chemotherapeutic explorations Because of their intriguing physicochemical properties, coupled with their unique biological behavior, the heterocyclic N-oxides have earned the separate classification as an independent, unique family of organic compounds.66-73
Conclusion and Future Work
The synthesis of the target imidazole derivative, 5-dichloromethyl-1-p-methoxybenzyl-
4-nitroimidazole (II), has been accomplished. Compound II
is a key imidazole precursor for the synthesis of a wide variety of old as well as new
"fat" nucleobases and nucleosides. The synthesis of II is
short, convenient, reasonably good-yielding, and can be prepared on a large scale from
readily available and inexpensive starting materials. Furthermore, the dichloromethyl
functionality of II is anticipated to be amenable for easy conversion
into a number of other reactive functional groups including, but not limited to, a
carboxaldehyde, a carboxylic acid, aor an iminomethylene group. We have further
demonstrated the use of II in future "fat" nucleoside syntheses
by successfully synthesizing a representative "fat" nucleobase III.
Acknowledgment
This work was supported by a grant (# RO1 CA71079) from the National Institutes of Health.
References and Notes
(1) Rajappan, V.; Hosmane, R. S., "Investigations into Biochemical Mode of Inhibition of Guanase by Azepinomycin: Synthesis and Biochemical Screening of Several Analogues of Azepinomycin," Nucleosides & Nucleotides 1999, 18, 835-836.
(2) Bretner, M.; Beckett, D.; Sood, R. K.; Hosmane, R. S.,"Substrate/Inhibition Studies of Bacteriophage T7 RNA Polymerase by the 5'-Triphosphate Derivative of a Ring-Expanded ("Fat") Nucleoside Possessing a Potent Anti-Hepatitis B Viral Activity," Bioorg. Med. Chem. 1999, in press.
(3) Bretner, M.; Beckett, D.; Hosmane, R. S., "Potent Anti-Hepatitis B Viral Activity and Inhibition of Bacteriophage T7 RNA Polymerase by a 'Fat" Nucleoside and Its 5'-Triphosphate Derivative: Synthetic, Biochemical, and Biological Studies of 4,8-Diamino-6-imino-6H-1-?-D-ribofuranosylimidazo[4,5-e][1,3]diazepine-5'-triphosphate," Nucleosides & Nucleotides 1999, 18, 837-838.
(4) Chen, H.; Sood, R.; Hosmane, R. S.,"An Efficient, Short Synthesis of 6-Imino-6-H-1-?-D-ribofuranosyl-4,5,7,8-tetrahydroimidazo[4,5-e]diazepine-4,8-dione: A Novel Ring-Expanded Purine Nucleoside Analogue Containing a 5:7-Fused, Planar, Aromatic, Heterocyclic Ring System," Nucleosides & Nucleotides 1999, 18, 331-335.
(5) Rajappan, V. P.; Hosmane, R. S., "Synthesis and Guanase Inhibition Studies of a Ring-Expanded Purine Analogue Containing a 5:7-Fused, Planar, Aromatic Heterocyclic Ring System," Bioorg. Med. Chem. Lett. 1998, 8, 3649-3652.
(6) Rajappan, V. P.; Hosmane, R. S., "Analogues of Azepinomcin as Inhibitors of Guanase," Nucleosides & Nucleotides 1998, 17, 1141-1151.
(7) Hosmane, R. S.; Hong, M., "How Important Is the N-3 Sugar Moiety in the Tight-Binding Interaction of Coformycin with Adenosine Deaminase?" Biochem. Biophys. Res. Commun. 1997, 236, 88-93.
(8) Hong, M.; Hosmane, R. S., "Irreversible, Tight-Binding Inhibition of Adenosine Deaminase by Coformycins: Inhibitor Structural Features that Contribute to the Mode of Inhibition, " Nucleosides and Nucleotides 1997, 16, 1053-1057.
(9) Bhan, A.; Hosmane, R. S., "Analogues of Azepinomycin: Inhibitors of Guanase," Nucleosides and Nucleotides 1995, 14, 455-458.
(10) Bhan, A.; Hosmane, R. S.; Zhang, H.; Hosmane, N. S., "A Diaminomalonate Synthon Useful for Building Novel Heterocycles," Synth. Commun. 1995, 25, 2723-2737.
(11) Bhan, A.; Hosmane, R. S., "Novel Inhibitors of Guanase," Tetrahedron Lett. 1994, 35, 6831-6834
(12) Wang, L.; Bhan, A.; Hosmane, R. S., "A Short Synthesis of a Novel Ring-Expanded Purine and Its Nucleoside Analogue Containing the Imidazo[4,5-e][1,3]diazepine Ring Skeleton with Multiple Amino Substituents Attached to the 7-Membered Ring," Nucleosides and Nucleotides 1994, 13, 2307-2320.
(13) Ostrowski, S. Synthesis of Some New Imidazole Derivatives. Polish J. Chem. 1994, 68, 2237-2247.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0029 as the message subject of your e-mail.