
[B0003]
Single bead FTIR in the optimization of solid-phase combinatorial organic syntheses
Bing Yan
Novartis Pharmaceuticals Corporation,
556 Morris Avenue, Summit, NJ 07901
Tel: 908-277-6023, Fax: 908-277-2785, E-mail: [email protected]
Received: 10 August 1999 / Uploaded: 10 August 1999Abstract:
The role of single bead FTIR in the reaction optimization stage of solid-phase combinatorial organic synthesis is reviewed. Keywords: Solid-phase organic synthesis, Combinatorial Chemistry, FTIR, Single bead.Introduction
Solution organic reactions need to be optimized on solid-phase before combinatorial synthesis1 using solid-phase organic synthesis (SPOS) method2. Selected reactions selected to synthesize a combinatorial library are usually optimized in solution so that the corresponding SPOS reactions would usually lead to expected products. Consequently, the analytical task is only to confirm the presence of the desired products. Single bead FTIR is particularly suited for this task because: 1) IR easily monitors functional group transformation; 2) building blocks used in synthesis can be selected to contain an IR detectable group; 3) direct monitoring of compounds on solid phase is generally quicker and more convenient than methods requiring cleavage and it is particularly advantageous when synthetic intermediates are unstable to the necessary cleavage conditions; 4) a single bead was shown representative of the whole population of beads;3a 5) IR spectra taken from different beads (different sizes or at different reaction times) can be compared using a polystyrene band as internal reference. Therefore, it is not necessary to examine the same bead for reaction kinetics study in the course of a synthesis.
Results and Discussion
The single bead FTIR method has been applied to optimize SPOS in every steps from beginning to end. Examples are given below.

The First Step – Loading the Resin. The attachment of the starting molecule species to solid support is a fundamental step in SPOS. It is important that this step proceeds to completion. Commonly used resins often utilize chloromethyl or hydroxymethyl groups as linkers. The completion of the loading reaction for chloromethyl group can be determined by chlorine elemental analysis5 or IR (1265 cm-1) while the completion of Figure 1. (A) Single bead IR spectra at various times during the synthesis of 2. One drop of resin suspension was taken out of the reaction mixture at various times and washed with DCM. The microscope was adjusted to focus on a single bead and spectra were taken as previously described. (B) The kinetics of the reaction in this reaction.
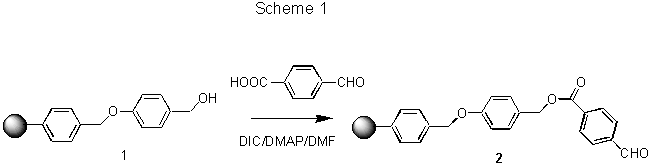
Reactions starting with the hydroxymethyl groups can be confirmed using FTIR by monitoring vibrations of the hydroxyl bond (for example see Scheme 1 and Fig.1A). Reactions with carboxylic acid and aldehyde resins can be monitored by confirming the disappearance of their respective carbonyl band.
In-Process Monitoring and Reaction Condition Optimization.
Single bead FTIR has been used extensively in solid-phase reaction optimization and its usefulness has been attested in the following aspects.Quick Yes-or-No Answer. A single bead FTIR analysis, comparable to TLC analysis, can be rapidly performed at any time during synthesis to give a qualitative answer to the reaction being monitored.6
Solid-phase Reaction Kinetics. Single bead FTIR has been effectively used in the study of reaction kinetics.7,8,10 An example (Scheme 1) is shown in Fig.2B.
Solid support. Reaction conditions and rates vary among solid supports. Kinetics studies on different supports using single bead FTIR have discovered many exceptions of common perceptions auch as that reactions on the PS-PEG resin are always faster than those on PS resins. As expected, the effects of polymer matrix on the reaction rate were found to be at least as complex as the solvent effects encountered in solution-phase reactions and no single polymer support is best suited for all reactions/conditions. A comparison of reaction rate on PS and PS-PEG resins (Scheme 2)8 is shown in Fig.2.
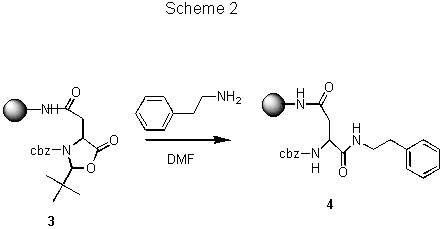
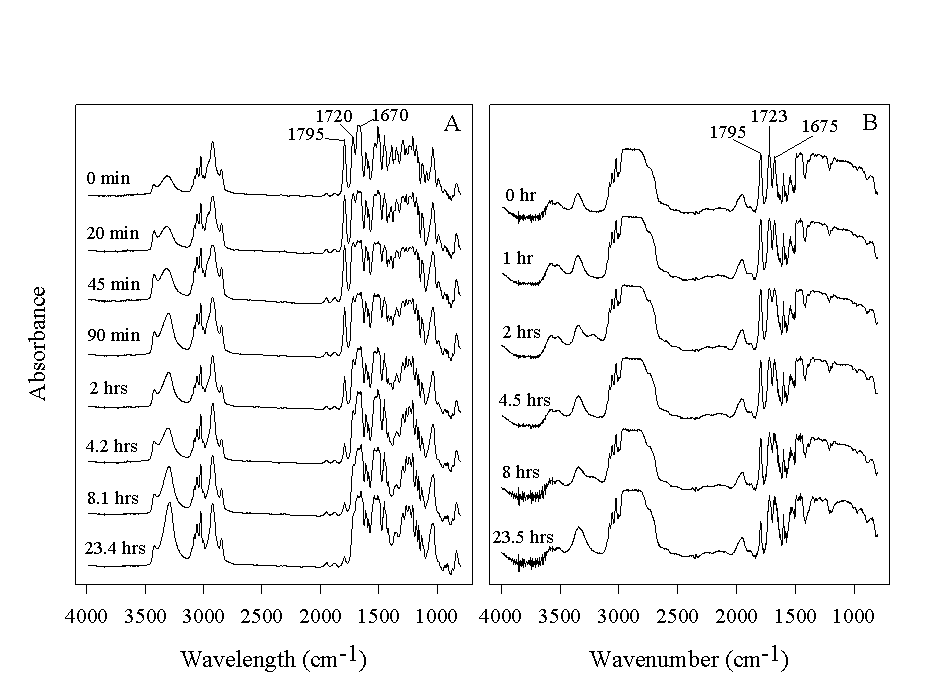
Figure 2. IR spectra taken from a single flattened PS bead (A) or a single PEG-PS bead (B) at various times during the reaction in Scheme 2.
Solvents effects on SPOS. Solvent plays multiple roles in SPOS, e.g. swelling of the polymer support, stabilizing the transition state, and solubilizing the reagents and reactants. Poor solvent selection gives rise to complex and poorly reproducible results. Solvent comparison studies using single bead FTIR have been routinely performed.
Mixing/Agitation. Based on single bead FTIR study of a SPOS reaction,9 the rotation and nitrogen bubbling methods provided the highest mixing efficiency. Mixing with an orbit shaker did not give satisfactory reaction yield compared with other mixing methods. However, excess of reagents and increased reaction times could in general drive the reaction to completion regardless of method of agitation.
Catalysts. Only soluble catalysts are suitable for SPOS. Larger amounts of catalysts are usually required in SPOS as compared with solution phase methods. For example, the rate constants for a catalytic oxidation of an alcohol to an aldehyde were determined to be 4.61x10-4, 1.64x10-4, and 1.18x10-4 s-1 using 0.2, 0.1, and 0.05 eq. of catalyst.10
The Last Step - Cleavage. The cleavage of compounds from the solid support using an optimal cleavage reaction is critical for the purity and the yield of the final library product.
The TFA cleavage kinetics of 16 resin-bound carbamates, ureas, amides, and sulfonamides from four different acid-labile linkers have been compared.11 Results showed that required TFA concentration (0.5-5 %) is generally lower and the time is shorter (5 min-3hrs) than those commonly reported in the literatures (5-30 %, 4-10 hrs).Summary
Direct single bead FTIR analysis is a simple, sensitive, fast and highly reliable method. It offers a way of monitoring reactions on solid support without stopping them or cleaving product from the resin and often provides information that would be hard to obtain in any other way. IR peak shift and peak area changes can be used to observe and quantitate intermediates in SPOS. Single bead FTIR can also provide quantitative and kinetic information. Therefore, it is an effective analytical tool in the process of transferring solution-phase reactions to solid phase and optimizing SPOS reactions.
Acknowledgments
I acknowledge significant contributions from my coworkers listed as co-authors in references 3-11.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with B0003 as the message subject of your e-mail.