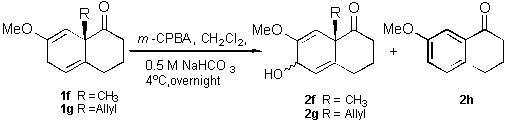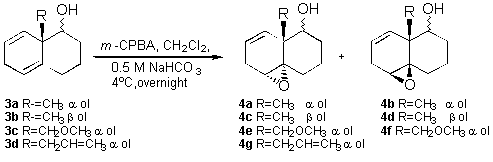Fourth International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0033]
EPOXIDATION
STUDIES OF DECALIN-1,4-DIENONES AND RELATED ALCOHOLS
Received: 25
July 2000 / Uploaded: 29 July 2000
Abstract: The
regio- and diastereoselectivities of epoxidations of decalin-1,4
-dienones and dienols obtained from the Birch reduction
alkylation of different a-tetralones
is described. They appear to depend on the steric approach
control of the peracid and show an important contribution of the
directing effect of homoallylic alcohols.
Introduction
In connection with our project dealing with the development of
synthetic intermediates to be utilized in the synthesis of
natural products,1 we decided to explore the regio and
stereoselectivity of the epoxidation of 1,4-dienes generated from
the Birch reductive alkylation reaction of substituted a-tetralones. We choose m-chloroperbenzoic
acid (m-CPBA) over dimethyldioxirane (DMDO), to carry the
epoxidation, due to its previously reported2 better
stereocontrol. In spite of the well-known sensitivity of the
products it was necessary to select very carefully the reaction
conditions in order to minimize the undesired rearrangements.
Results and Discussion
We used the dienone 1a to optimize the procedure and
tried mainly basic media due to the characteristics of the
substrate (m-CPBA, CH2Cl2, -20 �C with or without NaHCO3
(s);3 m-CPBA, CHCl3, Na2CO3
(s), 0 �C;4 MMPP, CH2Cl2,
RT;5 all produced low yields and re-aromatization
products). The best results were obtained when m-CPBA was
used under buffered heterogeneous system CH2Cl2-0,5
M NaHCO3 (pH = 8.3) at 4 �C6
or, even better, with a phosphate buffer (pH= 8.0)7
and were used for dienones 1a-e as shown in Scheme 1.
The dienones (1a-e) were prepared according to our
previous publication.8
Scheme 1
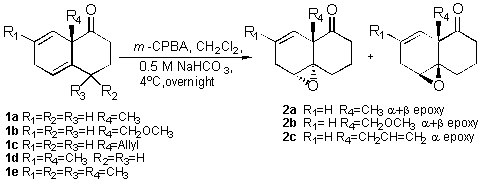
The dienone 1a, as expected according to the literature
precedents,9,10 reacted regioselectively with the more
substituted double-bond producing a diasteroisomeric a:b mixture of the mono epoxides in a 2.7:1
ratio. The moderate stereoselectivity found could be explained by
the shape of the dienone, which is almost planar, with an axial
substituent creating a steric impediment and making more
difficult the approach of the peroxide on this face.
Table 1 resumes the results obtained
for all the ketones. As can be seen, the reaction with the
dienone 1b occurs with the same stereo and regio
selectivity as for the dienone 1a. In the case of the
dienone 1c, the stereoselectivity was complete.
Table 1: Results of
the epoxidation of the 1,4 dienones
Compounds
|
Products
|
| |
|
a : b ratio
|
% yield
|
1a
|
2aa
+ 2ab
|
2.7 : 1
|
60
|
1b
|
2ba
+ 2bb
|
2.7 : 1
|
72
|
1c
|
2ca
|
1 : 0
|
62
|
1d
|
Complex mixtures of
mono and di-epoxides
|
1e
|
Complex mixtures of
mono and di-epoxides
|
1f
|
2fa + 2fb
|
1.6 : 1
|
32
|
1g
|
2ga + 2gb
|
1.8 :1
|
35
|
Surprisingly, dienone 1f under the previously described
conditions produced the alpha allylic alcohol 2fa (20 %), the beta allylic alcohol 2fa (12 %) and the re-aromatization
product 2h (63 %). In turn, from the dienone 1g, we
isolated the alpha allylic alcohol 2ga
(22,5 %), the beta allylic alcohol 2gb
(12,5 %) and the re-aromatization product 2h
(54 %) (Scheme 2).
Scheme 2
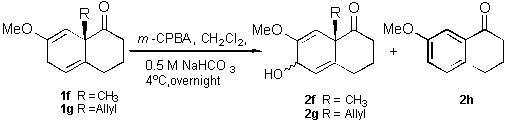
In an attempt to improve the diastereoselectivity of the
epoxidation, we devoted some efforts to study the stereochemical
course of the epoxidation of the dienols, in order to determine
the importance of the hydroxylic group directing effect toward
the peracid approach. The alcohols were synthesized by selective
reduction of the ketone8 and epoxidized using the same
conditions discribed for the ketones (Scheme 3).
Scheme 3
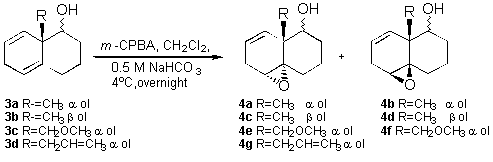
Table
2: Results of the epoxidation on the 1,4 dienols
Compound
|
Products
|
| |
a
epoxide
|
b
epoxide
|
a : b ratio
|
% yield
|
3a
|
4a
|
4b
|
4.7 : 1
|
90
|
3b
|
4c
|
4d
|
1.5 : 1
|
71
|
3c
|
4e
|
4f
|
10 : 1
|
82
|
3d
|
4g
|
|
1 : 0
|
64
|
Table 2, shows that the homoallylic a-alcohols
(3a and 3b) have a noticeable directing effect,
over the peracid, improving the stereoselectivity of the addition
by two or three folds compared with that of the related ketone.
On the other hand the b-alcohols
produced a lower diasteroselectivity. This directing effect had
been observed in homoallylic alcohols,11 but it had
not been reported for these 1,4 dienols systems. As expected, for
3d the alpha epoxide was the only product obtained.
The relative stereochemistry of the epoxy alcohols was
determined based on the analysis of its 1H NMR
spectra. The observation of a coupling between C-1 methine proton
and C1-OH hydrogen was a clear indication that this hydrogen is
involved in an intramolecular hydrogen bond and therefore this is
a strong evidence of their relative orientations.
Summary and Conclusions
- We studied the epoxidation reaction of the dienones
products of Birch alkylation reaction of a-tetralones and how non-bonding
interactions affect the stereochemical course of the
reaction.
- The complete regioselectivity observed is based on the
greater rate of the reaction of trisubstituted doble
bounds.
- We also established how the nature of the axial
substituent of the dienone affects the selectivity of the
reaction.
- We have examined the directing effect of different
homoallylic alcohols and, as expected, a substantial
preference toward the alpha epoxide for alpha alcohols
was observed.
References
- (a) Vila, A. J.; Cravero, R. M.;
Gonz�lez-Sierra, M. Tetrahedron Lett 1991,
32, 1929-1932. (b) Vila, A. J.; Cravero, R. M.;
Gonz�lez-Sierra, M. Tetrahedron 1993, 49,
4511-4526, (c) Labadie, G. R.; Cravero, R. M.;
Gonz�lez-Sierra, M. Synth. Comm. 1996, 26,
4671-4684.
- Schultz, A. G.; Harrington, R. E., Tham,
F. S. Tetrahedron Lett. 1992, 33,
6097-6100.
- La Clair, J. J. ; Lansbury, P. T.; Zhi,
B-X.; Hoogsteen, K. J. J. Org. Chem. 1995,
60, 4822-4833.
- Van Hifte, L.; Little, R. D.; Peterson, J.
L.; Moeller, K. D.; J. Org. Chem. 1987, 52,
4647-4761.
- Brougham, P.; Cooper, M. S.; Cummerson, D.
A.; Heaney, H.; Thompson, N. Synthesis, 1987,
1015-1017.
- Anderson, W. K.; Veysoglu, T. J. Org.
Chem. 1973, 38, 2267-2268.
- Ituma, M.; Ziffer, H. J. Org. Chem.
1979, 44, 1351-1352.
- Labadie, G. R.; Cravero, R. M.;
Gonz�lez-Sierra, M. Synth. Comm. In press
- Paquette, L. A. ; Barett, J. H. Org.
Synth. 1969, 49, 62-65.
- (a) Heatcock, C. H.; Amano, Y. Tetrahedron
1968, 24, 4917-4921; (b) Holbert, G. W.; Ganem,
B.; Engen, D. V.; Clardy, J.; Borsub, L.; Chantrapromma,
K.; Sadavongvivad, C.; Thebtaranonth, Y. Tetrahedron
Lett. 1979, 43, 715-719.
- Hoveyda, A. H.; Evans, D.A.; Fu, G. C. Chem.
Rev. 1993, 93, 1307-1370.
All comments on this poster should be sent by e-mail to (mailto:[email protected]
ona.edu)
[email protected]
with A0033 as the message subject of your e-mail.