2. Synthesis of Carbonyl O-oxides
Diazo compounds 3a and 3b were irradiated with l > 515 and 495 nm in oxygen saturated CCl3F or in 1 : 1 mixtures of CCl3F/(CBrF2)2 at low temperatures. The absorption maxima of the intense yellow solutions were at 398 and 394 nm. The yield of 5a is estimated to 20 - 25 %. As expected, 5a is very photolabile and irradiation with l > 475 nm rapidly results in the decolorization of the solution and formation of dioxirane 6a as the major product (Scheme 1) 10, 12. Alternatively, carbonyl oxide 5a was synthesized by the reaction of diazo compound 3a with 1O2 in a variety of solvents with small amounts of photosensitizers. Good results were obtained in CCl3F / octafluoroporphyrin, THF / rose bengal, or pentane / C60 at temperatures below -80° C and l > 570 nm excitation. Carbonyl oxide 5a is the first carbonyl oxide that could be characterized by 1H and - after 13C isotopic labeling - 13C NMR spectroscopy. The chemical shift of the "carbonyl" carbon atom of 5a was found at 211.0 ppm 10.
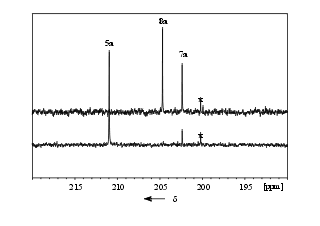
![]()
Figure 1:
13C-NMR spectra showing the thermal reaction of 5a to
produce 7a and 8a.
Bottom: Spectrum produced by
irradiation
(l > 515 nm) of 13C-3a in
O2 saturated CFCl3/(CBrF2)2
at 77K and subsequent warming to -70° C.
Top: Same as bottom,
but additional warming to room temperature and re-cooling to -70° C.