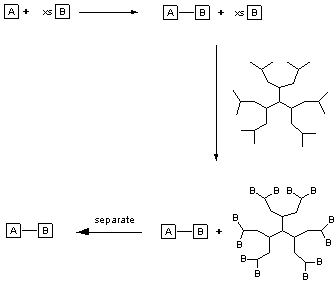
Scheme 1
[B0011]
Lorenzo Williams* and Siren M. Neset
SINTEF Applied Chemistry, P.O. Box 124 Blindern, N-0314 Oslo, Norway.
Received: 31 August 2000 / Uploaded: 31 August
Abstract: PAMAM dendrimers have been used to scavenge unreacted and excess reagents in synthesis.
Introduction
Polymer supported reagents /scavengers are widely used in synthesis. However, the supports themselves often vary in quality from batch to batch. This leads to unpredictability in reaction kinetics and variable swelling behaviour, in addition to problems which may arise due to the presence of contaminants from the polymerisation process. In an attempt to bypass these problems soluble supports have been developed, though they tend to suffer from very low loadings.1 Dendrimer supported reagents /scavengers may avoid these shortcomings.
Dendrimers and hyperbranched polymers have previously been used as soluble supports in combinatorial synthesis.2 We hereby present preliminary results for the use of dendrimers as scavengers in organic synthesis.
Results and Discussion
Dendrimers are tree-like molecules, often composed of a central core containing many branches. It was anticipated that they could be used in synthesis as sequestering agents in order to capture unreacted or excess reagents, products or by-products. For example a dendrimer containing the appropriate functionality at each terminus could be used in the capture of excess reagents used to drive a reaction to completion (Scheme 1). It is preferable that the dendrimer be removed in a simple and straightforward fashion, e.g. by precipitation and filtration.

Scheme 1
Commercially available Starburst polyamidoamine (PAMAM) dendrimers were considered as sequestering agents for our needs. PAMAM generation 4.0 was initially chosen due to it's high loading, containing 64 surface primary amino groups (Fig 1). A variety of PAMAM dendrimers are currently available, and although they contain little variation in functionality their flexible framework should allow for easy access to other types of scavenger via simple chemical transformation.
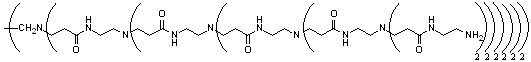
Fig 1 Polyamidoamine dendrimer generation 4.0 with 64 terminal amino groups
As model reactions the reaction of an arylpiperazine with an isocyanate, sulfonyl chloride and a benzyl halide were considered (Scheme 2). In each case the arylpiperazine was reacted with a slight excess of the electrophile (1.2 eq.). In the reactions with the sulfonyl chloride and benzyl halide, pyridine (1.2 eq.) was used as base and to initially scavenge HCl or HBr liberated in these reactions. PAMAM dendrimer generation 4.0 was utilised as scavenger after TLC analysis indicated complete consumption of the starting amine. The dendrimer was added as a 10 % solution in methanol and the reaction stirred for a further 1 hour. Solvent was then removed in vacuo and the residue taken up in chloroform. Filtration of the insoluble dendrimer and concentration of the filtrate afforded the products in high yield and excellent purity.
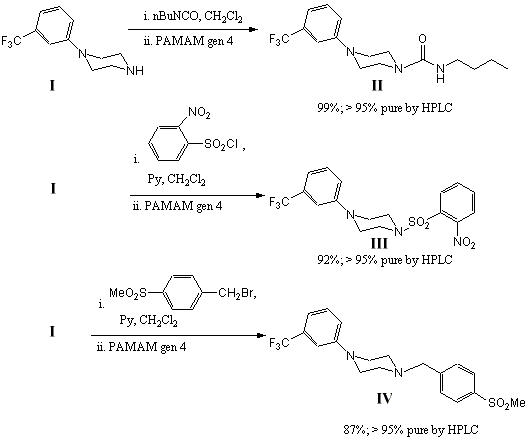
Scheme 2
The use of dendrimers as scavengers has many advantages over conventional scavenger resins. Sequestering reactions occur rapidly since the dendrimers are soluble and reaction occurs in solution as opposed to a solid-liquid interface. The larger generation dendrimers contain a considerable number of functional groups giving rise to quite high loadings. Furthermore the use of dendrimers as scavengers is far more readily amenable to automation as the dendrimer can be added in solution, thereby avoiding tedious preweighing procedures.
Conclusion
PAMAM dendrimers proved to be effective scavengers
of isocyanates, sulfonyl chlorides and benzyl bromides leading to the facile
isolation of products in high yields and purity. The simplicity of the
method lends itself to use in parallel synthesis for the rapid purification
of large numbers of compounds. Investigations towards this end are currently
in progress.
All comments on this poster should be sent by e-mail to (mailto:[email protected]) [email protected] with B0011 as the message subject of your e-mail.