Synthesis and application of a new polymer-supported anhydride as scavenger resin for solution-phase chemistry
School of Pharmaceutical Sciences,University of Nottingham, University Park, Nottingham NG7 2RD, UK and ¶CN Biosciences (UK) Ltd., Boulevard Industrial Park, Beeston, Nottingham NG9 2JR, U.K.
Received: 20 August 2001 / Uploaded 21 August 2001
Introduction
Following the successful introduction and extensive development of solid-phase peptide and organic syntheses,1 attention is focused in recent years in the development of robust polymer-supported reagents for use in traditional solution-phase reactions. These polymer-supported reagents typically function either as catalysts or scavengers.2 This combination of solution-phase with solid-phase reagents allow the removal of excess reagents and by-products by simple filtration methods without the need for chromatographic purification.
Results & Discussion
We herein report a new high-loading carboxy-anhydride scavenger resin, and its properties and applications in solution-phase chemistry are described. The new reagent 1 display the advantages of combining a polymer-supported structure with a high substitution level (4.0-4.32 mmol g-1). In addition, the macroporous structure of the resin results in low swelling properties (1.8-3.4 ml g-1 in CH2Cl2, MePh, THF, DMF, i-PrOH, MeOH) and compatibility with most solvents used in organic chemistry. Such physical properties, together with its high substitution level, make this resin an effective and robust scavenger for solution-phase synthesis.
![]()
Polymer-supported reagent 1 was characterised by IR spectroscopy (nmax: 1759, 1805 cm-1), whilst its loading (4.32 mmol g-1) was determined by exposure to excess n-propylamine followed by elemental analysis of the resultant resin product 2 (Scheme 1).
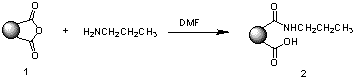
Scheme 1. Reaction of anhydride resin 1 with propylamine
As illustrated in Scheme 1, nucleophilic attack of the amine at the cyclic anhydride results in amide bond formation via ring opening of the anhydride 1. The reactivity of the resin 1 towards primary, secondary and aromatic amines was then further investigated, which established the efficient scavenging properties of the resin (Table 1). Typically, a two-fold excess of resin over a 2-4 hour reaction period was required for the complete removal of non-hindered primary and secondary amines. In contrast, scavenging of substituted anilines was effected with longer reaction times at elevated temperatures (18 h at 60C). As anticipated, the resin 1 was found to be inefficient for the complete removal of severely hindered amines, e.g. N, N-diisopropylamine.