Fifth International Electronic Conference
on Synthetic Organic Chemistry (ECSOC-5), http://www.mdpi.org/ecsoc-5.htm, 1-30
September 2001
[B0008]
A Powerful Tool for Generic Fluorescence
Labelling of Combinatorial Compound Libraries
Hubert Gstach,
Karin Pflugseder, Susanne Prechelmacher (née Schmidt), Daphne Monteux, Simon
Eliot Lewis, Thomas Storz, Jörg Steffen Früchtel, Christine Graf, Arno Pruckner
and Manfred Auer

Novartis Forschungsinstitut Wien G.m.b.H, Brunnerstrasse 59, A-1235 Vienna,
Austria
Received: 23 August 2001 / Uploaded 23 August 2001
CONTENTS:
INTRODUCTION
GENERAL PROPERTIES OF
THE FLUORESCENT AIDA-DYES
SPECTROSCOPIC
FEATURES OF AIDA-DYES
GENERAL
SYNTHESIS
SPECIFIC EXAMPLE:
AIDA-1
SYNTHESIS OF
AIDA-LABELLED LIBRARIES
CONCLUSION
EXPERIMENTAL
PROCEDURE
ACKNOWLEDGEMENTS
REFERENCES
INTRODUCTION
High throughput screening has become a discipline assimilating
synthetic chemistry, biochemistry, biophysics and molecular biology combined
with miniaturized detection/liquid handling technologies and automation
processes. Additionally, fluorescence spectroscopy has evolved to a routinely
used ultra-sensitive detection technology on modern screening platforms. An
integration of combinatorial chemistry and fluorescence spectroscopy creates a
powerful tool to probe biological targets.
The integration can be done on the chemical synthesis level by
providing generically labelled compound libraries. Especially, labelled target
molecules of unknown function (orphan targets) can be tested for their binding
affinity. This aspect forms the clamp between combinatorial chemistry and
functional genomics.
We report on the chemistry and optical spectroscopy of new
fluorophores for application in combinatorial chemistry and high throughput
screening in solution and on the solid support. The chemistry of these
fluorophores is generally described as AIDA-chemistry (An Indazole
based Discovery and Analysis Tool).1
GENERAL PROPERTIES
OF THE FLUORESCENT AIDA-DYES
AIDA can
accept fluorescence resonance energy from natural protein fluorescence
AIDA can donate fluorescence
resonance energy to labelled targets
AIDA belongs to a class of
chemically very stable fluorophores
AIDA can be immobilised on solid
support
AIDA on solid support can serve as
starting point for combinatorial chemistry
AIDA is a generic fluorescence label
of compound libraries
AIDA-dyes as well as
compounds conjugated via a spacer element to the AIDA-dyes emit blue
fluorescence over a broad spectral range on excitation at their absorption
wavelength (350 nm). These fluorescence properties allow multiple applications
in fluorescence based processes for the identification of molecular interactions
and for the recognition of molecules which bind to target molecules like
peptides, proteins, nucleic acids, carbohydrates etc. The fluorescence detection
technologies used for monitoring binding of AIDA-conjugated molecules include
conventional macroscopic techniques (ensemble averaging) which detect changes in
fluorescence intensity, anisotropy (polarization), fluorescence resonance energy
transfer, fluorescence lifetime, rotational correlation time as well as single
molecule spectroscopic techniques. The dyes show high stability in chemical
transformations. The indazole core of the dyes is not photoreactive under the
conditions used in cleavage of compounds from the solid support and detection of
binding events to target molecules, respectively. These features make AIDA-dyes
an excellent tool for combinatorial chemistry (solid phase and solution phase
chemistry) and biological investigations (ultra high throughput
screening).
SPECTROSCOPIC
FEATURES OF AIDA-DYES
To prove the spectroscopic responses of AIDA-conjugates, we
have synthesised AIDA-biotin conjugates in solution as well as on solid support.
The biotin was attached to the core of the chromophor by different spacers and
on different positions of the phenyl substituents.
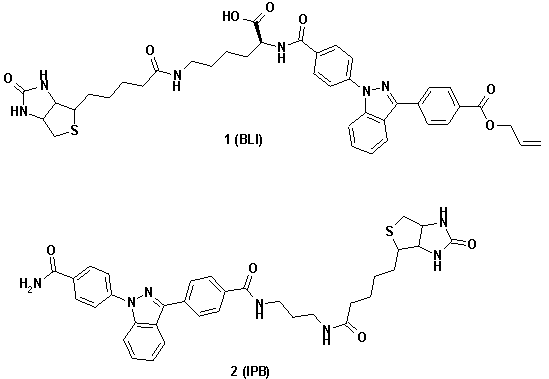
The excitation spectra of derivatives 1 and 2
revealed a nearly perfect overlap of their absorption bands with the emission of
tryptophan. This feature of AIDA establishes the physical basis to act as
acceptor for natural protein fluorescence (figures 1a-b). The corresponding
emission spectra of AIDA-conjugates 1 and 2 revealed overlap
integrals with the absorption spectra of commercial fluorescence labels (e.g.
BODIPY-FL™, figures 1c-d) establishing the donor properties of
AIDA.
Figure 1.
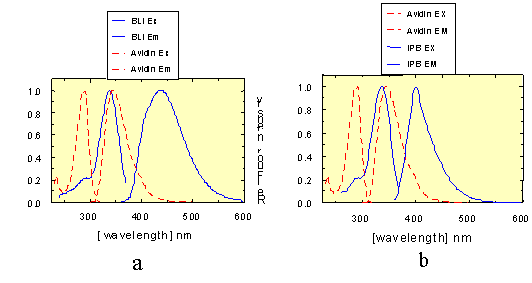
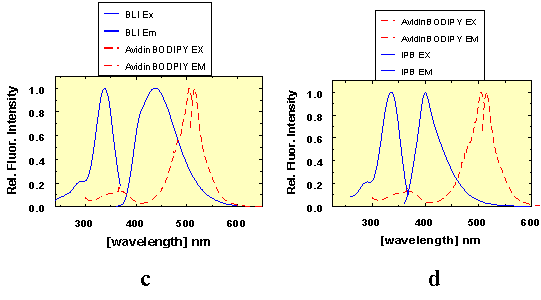
(1a)-(1d): Excitation and emission spectra of AIDA-biotin
conjugates 1 and 2 (in the graphical representations assigned as
BLI and IPB, respectively), and avidin-tryptophan (1a-b) and
avidin-BODIPY-FL™ (1c-d), showing the spectral overlap between
tryptophan, BODIPY-FL™ emission and excitation, respectively.
Donor and Acceptor Properties of AIDA:
The avidin - biotin high affinity interaction was used to probe
the donor and acceptor qualities of AIDA-conjugate 1 (BLI) (figures 2 and
3).
(a) AIDA as an acceptor of natural protein fluorescence
(Figure 2)
Figure 2.
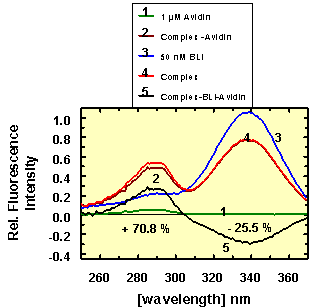
Figure 2 shows the fluorescence excitation spectra of 50 nM BLI (curve 3)
and complexed to 1 µM unlabeled avidin (curve 4). An emission wavelength at 480
nm was chosen because the tryptophan fluorescence of avidin had almost reached
the baseline level at this emission energy, resulting in only minor avidin
fluorescence contribution between 250 and 370 nm (curve 1). The difference
spectrum: [I(BLI (free) )+ I(Avidin (free) )] - I(BLI-Avidin complex ), shown in
black, indicates that the BLI fluorescence is quenched by about 25% by
complexation in the avidin binding site. This quenching effect can be attributed
to all radiative and non-radiative processes occurring to the BLI in the
vicinity of the tryptophans in the binding cleft. From 250 to 305 nm the
enhancement of the tryptophan and tyrosine fluorescence intensity is 70% based
on the sum of BLI and avidin (curve 5).
Conclusion:
When BLI is used as acceptor and protein fluorescence in avidin as donor,
both an energy transfer to, and a quenching effect on BLI can be used as a
signal for the complex formation.
(b) AIDA as a donor to BODIPY-FL™ labelled avidin (Figure
3):
Figure 3.
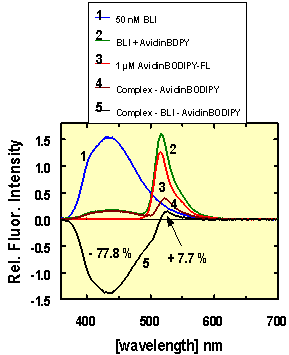
Figure 3 shows the fluorescence emission spectra of 50 nM BLI
(1, curve 1), 1 µM avidin-BODIPY™ (curve 3), the complex (curve
4) - and the relevant difference spectra (curve 5). In these experiments BLI was
used as donor and BODIPY™ as acceptor. Two excitation wavelengths, 338
nm (maximum) and 325 nm were used to test for BODIPY™ excitation at
the blue edge of the BLI (1) absorption spectrum. At both excitation
wavelengths, the BLI emission between 360 nm and 542 nm is strongly quenched by
78% in the complexed form. 8% of the emission energy of BLI (1) is
transferred to BODIPY-FL™.
Conclusion:
The energy transfer from AIDA-biotin conjugate 1 (BLI)
to avidin-BODIPY™ is weak. But the quenching effect of almost 80% on
BLI between 360 nm and 516 nm is an extremely efficient signal for biotin
interaction with avidin monitored by BLI at wavelengths between 440 nm and 500
nm.
GENERAL
SYNTHESIS:
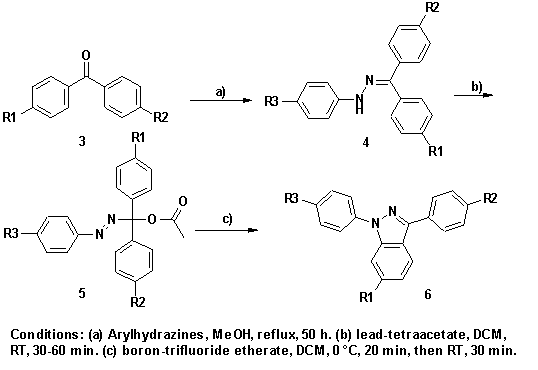
|
6 |
R1 |
R2 |
R3 |
Yield (%) |
|
a |
H |
CO2Me |
CO2H |
75 |
|
b |
H |
NO2 |
CO2H |
78 |
|
c |
H |
CO2H |
NO2 |
42 |
|
d |
Me |
NO2 |
CO2H |
80 |
Among the known possibilities to synthesise the indazole
heterocycle2 and
more specifically 1,3-diaryl-1H-indazoles, the methods described by Gladstone et
al.3
enable an efficient access to the desired compounds.
The synthesis starts from substituted benzophenones 3
which are first converted to arylhydrazones 4 by reaction with
substituted arylhydrazines in methanol. The corresponding benzophenone
arylhydrazones 4 are transformed to 1-(arylazo)-1,1-diarylmethyl acetic
esters 5 by oxidation with lead tetraacetate, following a procedure first
described by Iffland.4
The arylazo-acetates 5 finally undergo ring-closure to
indazoles 6 upon treatment with Lewis acids (e.g. boron trifluoride ethyl
etherate) and proceeds via an intermediate 1-aza-2-azoniaallene.5 The reaction
is highly regioselective for the geminal arylsubstituents of the
arylazo-acetates 5. Cyclisation does not occur into aromatic rings
bearing strong electron withdrawing substituents, such as carboxyl-, ester-,
amide-, or nitro groups, respectively.
The synthesis of such derivatives (see table for selected
examples) can be carried out without purification by chromatography and gives
the desired compounds in moderate to good yields. The final products are
chemically very stable under strong acidic and basic conditions.
Additional derivatives are accessible by aromatic electrophilic
substitution (e.g. nitration) on the position 5 of the indazole core or by
further modification of an already existing functional group (e.g. reduction of
a nitro group).
Functionalized 1,3-diarylsubstituted indazoles can also be
synthesised on solid support starting with immobilised arylhydrazones of
appropriate benzophenone derivatives.6
SPECIFIC EXAMPLE:
AIDA-1
For loading of solid supports with AIDA-labels and further
transformation to the labelled conjugates, bifunctional indazoles are converted
to protected amino acids. Compound 6a is synthesised from benzoyl benzoic
acid methylester and 4-hydrazino benzoic acid according to the general
procedure. Subsequent treatment with diaminopropane followed by a
Fmoc-protection gives compound 8 (AIDA-1) which can then be coupled to an
amino resin (preferred: TentaGel) bearing an acid labile or a photocleavable
linker.
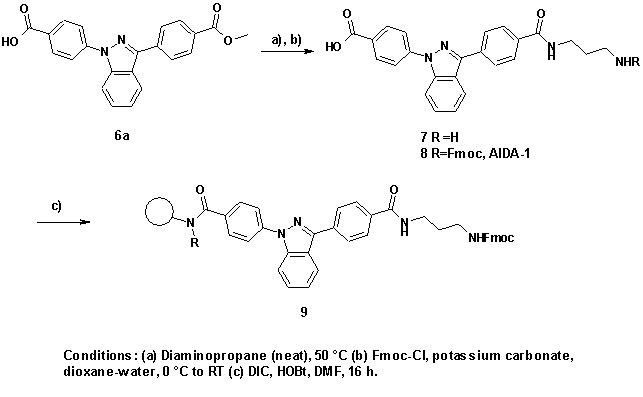
The diaminopropane moiety acts as a spacer providing a
protected functional group to start the synthesis of combinatorial compound
libraries.
SYNTHESIS OF
AIDA-LABELLED LIBRARIES
For the synthesis of AIDA-labelled on-bead-libraries, we follow
the split-and-mix strategy7 which is
particularly adapted for the production of very large numbers of
compounds.
The synthesized conjugates (one-bead-one-compound) have the
following general structure:
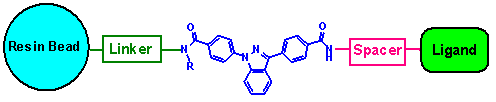
CONCLUSION
A generic fluoresence labelling tool (AIDA) for combinatorial
compound libraries has been designed. A versatile synthesis of a labelling
reagent to prepare resins for library synthesis has been created (AIDA-1 as a
Fmoc-protected amino acid derivative).
Up to now, several AIDA-1-labelled libraries have been
synthesized. The libraries were screened on-bead against various targets.
Subsequently, the spectroscopic features of the fluorescent label on the
compound were used to validate the primary on-bead binding of target molecules
in solution. The publication of the results is under preparation.
EXPERIMENTAL
PROCEDURE
Preparation of AIDA-1 loaded resin (TentaGel Rink Amide) for
library synthesis
4-{3-[4-(methoxycarbonyl)-phenyl]-1H-indazol-1yl}-benzoic
Acid (6a): was synthesised according to the literature3,4
4-{3-{4-[(3-Aminopropyl)-aminocarbonyl]-phenyl}-1H-indazol-1yl}-benzoic
Acid (7): 1,3-diamino-propane (80 mL) is added to 6a (10.0 g, 26.85
mmol). The heterogeneous reaction mixture is heated to 50 °C under stirring for
4h. The 1,3-diamino-propane is distilled off under reduced pressure giving a
viscous oil. The minimum amount of methanol is added to the crude product and
the desired compound precipitates. The crystals are collected by vacuum
filtration. The white crystals are washed with a portion of cold methanol and
then with several portions of diethyl ether. The product is dried under reduced
pressure, yielding 9.86 g (89 %) of 7.
(4-{3-{4-[N-3-[(9H-Fluoren-9-yl)-methyloxycarbonylamino]-propyl]-aminocarbonyl}-phenyl-1H-indazol-1-yl}
benzoic Acid (8): Potassium carbonate (2.67 g; 15.45 mmol) is added to a
suspension of the amino acid 7 (3.20 g; 7.73 mmol) in water (60 mL) and
1,4-dioxane (35 mL). The mixture is stirred for 5 min and cooled to 0
oC in an ice bath. Fmoc-chloride (2.20 g; 8.5 mmol) in 1,4-dioxane
(35 mL) is added dropwise within 10 min. Cooling is removed and stirring
continued at room temperature for 12 h. A bulky precipitate is formed. The
aqueous suspension is extracted with diethyl ether. The pH is adjusted to 1 with
diluted HCl and then further diluted with brine (100 mL). The precipitate is
collected by vacuum filtration and washed extensively with diethyl ether. The
product is dried under reduced pressure, yielding 4.14 g (84 %) of
8.
Coupling of the fluorescent dye reagent (8) onto the resin:
Deprotected TentaGel Rink amide resin is acylated with a 0.1 M solution of
8 (2 eq), 1-hydroxybenzotriazole (2 eq.), N,N´-diisopropyl carbodiimide
(4 eq.) in dimethylformamide overnight at room temperature. The resin is washed
thoroughly with several portions of dimethylformamide, then methanol, and
finally dichloromethane.
ACKNOWLEDGEMENTS
We thank the Andrea Steck's group (Novartis Vienna) for
structure confirmations by NMR-spectroscopy and Francis Roll (Novartis Basel)
for analysis by HRMS. We also thank Rocco Falchetto (Novartis Basel) for his
great efforts in decoding of single beads by µHPLC/MS.
REFERENCES
1. Auer M. and Gstach H. Fluorescent dyes (AIDA)
for solid phase and solution phase screening. PCT Int. Appl. (2000), WO 0037448.
Priority: US 98-217795 19981221.
2. for a review see: Elderfield, R. C. in
"Heterocyclic Compounds" ed. Elderfield, R.C., vol 5, John Wiley & Sons,
Inc., New York, 1957, p. 162.
3. Gladstone, W. A. F. and Norman, R. O. C. J.
Chem. Soc. (1965), 3048-52; Gladstone, W. A. F. and Norman, R. O. C. J. Chem.
Soc. (1965), 5177-82; Gladstone, W. A. F. et al. J. Chem. Soc. (1966),
1781-4.
4. Iffland, D. C. et al. J. Am. Chem. Soc.
(1961) 83, 747.
5. Wang, Q. et al. Synthesis (1992), 7,
710-8.
6. Yan, B. and Gstach, H. Tetrahedron Lett.
(1996), 37(46), 8325-8.
7. Furka, Arpad. The "Portioning-Mixing"
(split-mix) synthesis. Proc. ECSOC-1: First Int. Electron. Conf. Synth. Org.
Chem.; Proc. ECSOC-2: Second Int. Electron. Conf. Synth. Org. Chem.
(1999).
![]()








