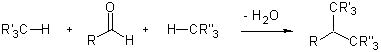
A Novel Three-Component Reaction
Benjamin List* and Chris Castello
Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA. [email protected], Fax: +858 784 7028, Phone: +858 784 7025
Received: 30 August 2001 / Uploaded 30 August 2001
The diversity generating potential of multicomponent reactions (MCR’s) has been recognized and their utility in preparing libraries to screen for functional molecules is well appreciated. Consequently, the design of novel MCR’s is an important field of research.1–3 We have recently developed an asymmetric aminocatalytic variant of the three-component Mannich reaction.4 Based on our experience with this efficient transformation, we decided to explore a related MCR, in which two C-H-bond-containing compounds condense with an aldehyde:5
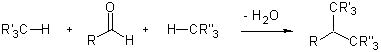
Symmetrical variants of this process include reactions of either two molecules of Knoevenagel nucleophiles or arenes with one molecule of aldehyde to furnish products of type A or B, respectively.6
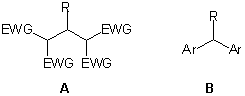
Unsymmetrical variants, in which two different C-H-bond containing compounds combine with an aldehyde are much less studied but useful for the creation of tertiary stereogenic centers.7,8 Herein we describe a novel proline-catalyzed three-component reaction of unmodified ketones with aldehydes and Meldrum’s acid. In this efficient and highly diastereoselective process a tertiary stereogenic center is created under concomitant formation of two new carbon-carbon s-bonds.
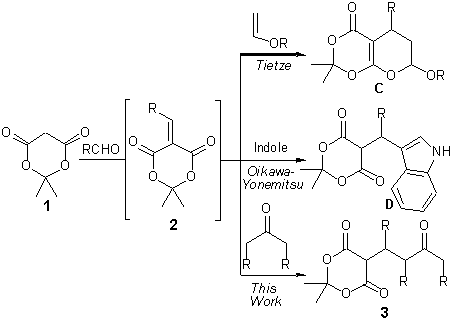
Our reaction design was inspired by elegant three-component reactions introduced by Tietze et al. and Oikawa and Yonemitsu et al. that utilize aldehydes, Meldrum’s acid (1), and enol ethers or indoles to furnish products of type C and D, respectively (Scheme 1).7,8 These transformations are catalyzed by ammonium salts or proline and follow a domino Knoevenagel-hetero-Diels-Alder mechanism via intermediate alkylidene Meldrum’s acids 2.
Furthermore, silyl enol ethersand
enamines are known to react with activated olefins (e.g. 2), to
give Michael-products (e.g. 3).9,10 We reasoned that
alkylidene derivatives 2 and enamines could be generated in
situ from ketones, aldehydes, and Meldrum’s acid (1) by using a
catalytic amount of proline. Consequently, this reaction mixture would directly
furnish keto esters 3.
Scheme 1. Three-Component Reactions Involving Aldehydes and Meldrum’s Acid.
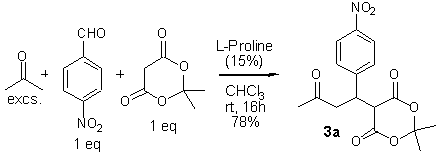
It was gratifying to find that mixing p-nitrobenzaldehyde (1 eq), Meldrum’s acid (1 eq), and acetone (excs.) with a catalytic amount of l-proline in chloroform at room temperature readily furnished crystalline keto ester 3a in 78% yield (Scheme 2).
Scheme 2.
Moreover we found that other aldehydes including a-branched (entry 4) and unbranched (entries 2,3) and cyclic aldehydes (entry 5) can readily be used to give the products in good yields (Table 1).
Table 1. Proline-Catalyzed
Three-Component Reaction of Aldehydes with Ketones and Meldrum’s Acid.
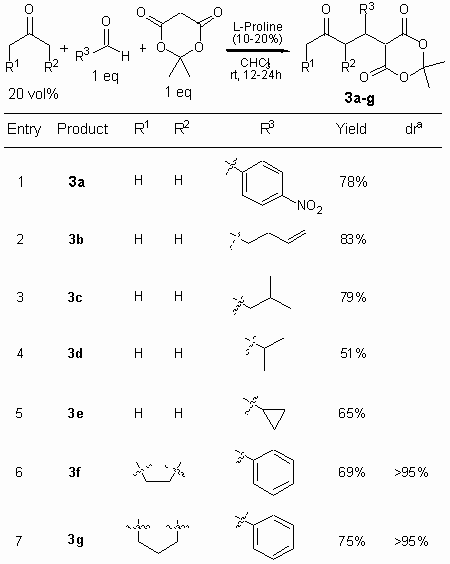
a Determined from HPLC
and NMR analyses.
Cyclic ketones may be used as well and the corresponding products (3f, 3g) are obtained as single diastereomers (entries 6,7). The enantioselectivity of this new reaction is generally low and keto esters 3 were typically obtained in ee’s under 5%. This result is consistent with our earlier experiments on proline-catalyzed Michael additions of ketones to nitroolefins, which showed low enantioselectivities.11 Presumably both proline-catalyzed transformations, Michael reaction and the present three-component reaction, have similar mechanisms, which however differ from the highly enantioselective proline-catalyzed aldol- and Mannich reactions.12-15,4
We propose that this new three-component reaction involves the domino Knoevenagel-hetero-Diels-Alder sequence postulated for the Tietze-three-component reaction (Scheme 3).7 In this sequence, proline utilizes both iminium- and enamine-catalysis.11 Accordingly, the initial Knoevenagel condensation (a-c) proceeds via iminium ion 7 and ammonium ion 8 to give olefin 2. The role of proline in the hetero-Diels-Alder step (d-f) is to generate the dienophile, enamine 9, which reacts with hetero diene 2 furnishing cyclo-adduct 10, and upon hydrolysis the final product (5) while regenerating the catalyst.
Scheme 3. Proposed Mechanism.
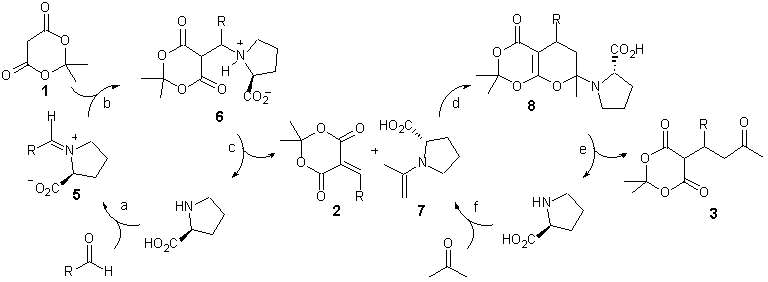
Although proline’s a-chirality is not utilized for asymmetric induction, the carboxylate functionality seems essential for catalysis as indicated by the fact that pyrrolidine is ineffective.
Meldrum’s acid derivatives 3 are valuable precursors of diversified 1,5-dicarbonyl compounds.8,9 This was demonstrated by methanolysis and in situ decarboxylation of compound 3b to give keto ester 4b in good yield (Scheme 4).
Scheme 4.
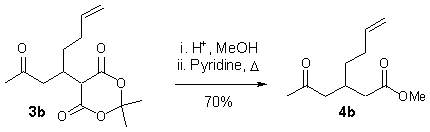
In summary we have developed a novel proline-catalyzed three-component reaction of aldehydes, ketones, and Meldrum’s acid. The reaction allows the rapid construction of complex carbon-scaffolds with two new C-C-s-bonds from three different commercially available components. From our initial experiments the scope of this new transformation appears to be broad since all aldehydes tested and various small and inexpensive cyclic and acyclic ketones could be used. Operational simplicity, mild reaction conditions, and the inexpensive and non-toxic catalyst proline all contribute to the overall usefulness of the process.
Our new reaction adds to an increasing
number of efficient organocatalytic transformations that are catalyzed by
small-molecule amines and amino acids.16 Future studies include the
search for novel enantioselective aminocatalysts for this three-component
reaction and the utilization of resin-bound Meldrum’s acid. We are also pursuing
the question whether additional substrates may be used to extend this novel
MCR17 beyond three components.
Acknowledgment. We thank Richard A.
Lerner and The Scripps Research Institute, for encouragement and generous
support and Peter Pojarliev for technical assistance.
References
(1) Tietze, L. F.; Beifuss, U. Angew. Chem. Int. Ed. Engl. 1993, 32, 131-163.
(2) Armstrong, R. W.; Combs, A. P.; Tempest, P. A.; Brown, S. D.; Keating, T. A. Acc. Chem. Res. 1996, 29, 123-131.
(3) (a) Dömling, A.; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168-3210. (b) Bienayme, H.; Hulme, C.; Oddon G.; Schmitt, P. Chem. Eur. J. 2000, 6, 3321-3329.
(4) List, B. J. Am. Chem. Soc. 2000, 122, 9336-9337.
(5) A transformation of this class formally represents a bis-alkyl(or aryl)-de-oxo-bisubstitution reaction. Because of close similarities to the acetalization, which is a bis-alkoxy-de-oxo-bisubstitution reaction, we refer to this process as carba-acetalization.
(6) (a) Tietze, L. F. in Comprehensive Organic Synthesis, Vol. 2 (Ed. B. M. Trost) Pergamon, Oxford, 1991, pp. 341-394. (b) An important industrial application of this reaction is the synthesis of DDT from trichloro-acetaldehyde and chloro-benzene, catalyzed by sulfuric acid.
(7) (a) Tietze, L. F. J. Hetereocyclic Chem. 1990, 27, 47-69. (b) Tietze, L. F. Chem. Rev. 1996, 96, 115-136. (c) Ref. 1 (d) For a recent example, see: Tietze, L. F.; Evers, T. H.; Töpken, E. Angew. Chem., Int. Ed. Engl. 2001, 40, 903-905.
(8) (a) Oikawa, Y.; Hirasawa, H.; Yonemitsu, O. Tetrahedron Lett. 1978, 1759-1762. (b) Oikawa, Y.; Hirasawa, H.; Yonemitsu, O. Chem. Pharm. Bull. 1982, 30, 3092-3096.
(9) Mizukami, S.; Kihara, N.; Endo, T. Tetrahedron Lett. 1993, 34, 7437-7440.
(0) Penades, S.; Kirsch, H.; Tortschanoff, K.; Margaretha, P.; Polansky, O. E. Monatsh. Chem. 1973, 104, 447-456.
(11) List, B.; Pojarliev, P.; Martin, H. J. Org. Lett. 2001, 3, 2423-2425.
(12) (a) Hajos, Z. G.; Parrish D. R. J. Org. Chem. 1974, 39, 1615-1621. (b) Eder, U.; Sauer, G.; Wiechert, R. Angew. Chem., Int. Ed. Engl. 1971, 10, 496-497.
(13) List, B.; Lerner, R. A.; Barbas C. F. III, J. Am. Chem. Soc. 2000, 122, 2395-2396.
(14) Notz, W.; List, B. J. Am. Chem. Soc. 2000, 122, 7386-7387.
(15) List, B.; Pojarliev, P.; Castello, C. Org. Lett. 2001, 3, 573-575.
(16) For other interesting recent examples, see: (a) Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243-4244. (b) Jen, W. S.; Wiener, J. J. M.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 9874-9875. (c) Paras, N. A.; MacMillan, D. W. C. J. Am. Chem. Soc. 2001, 123, 4370-4371. Also, see: (d) Gröger, H.; Wilken, J. Angew. Chem. Int. Ed. 2001, 40, 529-532 and references therein. (e) Albus, S. Nachr. Chem. Techn. Lab. 2000, 48, 1459. (f) Bahmanyar, S.; Houk K. N. Chemtracts 2000, 13, 904-911. (g) Diez, E.; Ley, S. Chemtracts 2000, 13, 592-595. (h) Doye, S. Chem. Unserer Zeit2001, 35, 62-63.
(17) The synthesis of 3b illustrates the general experimental procedure: 4-Pentenal (1.66 mL, 17 mmol), Meldrum’s acid (2.16 g, 15 mmol), acetone (30 mL), and l-proline (3 mmol, 20 mol%) were stirred in CHCl3 (120 mL) at room temperature for 12 hours. The mixture was washed with sat. aqueous ammonium chloride solution, and the aqueous layer was extracted with ethyl acetate. The combined organic layers were dried (MgSO4), filtered, and concentrated. Chromatography on SiO2 with hexanes/ethyl acetate (gradient 4:1 – 1:2) furnished product 3b (3.33 g, 83%). 1HNMR (250 MHz, CHCl3): d= 1.50 (m, 2H), 1.71 (s, 3H), 1.72 (s, 3H), 2.05 (m, 2H), 2.21 (s, 3H), 2.75 (dd, 1H, J= 3.3, 12.3 Hz), 2.88, (m, 1H), 2.97 (dd, 1H, J= 9.9, 12.3 Hz), 4.12 (d, 1H, J= 2.2), 4.96 (m, 2H), 5.72 (m, 1H). 13CNMR (75 MHz, CHCl3): d= 26.7, 28.2, 30.4, 31.5, 32.2, 43.7, 47.7, 104.8, 115.3, 137.5, 165.0, 165.1, 209.0. FT-IR (neat): 2924, 1746, 1301, 1206. HRMS (MALDI-FTMS): calcd for [M-H]- 267.1238, found 267.1231.