Rearrangement of ammonium ylides under microwave irradiation
Séverine Torchy, Guy Cordonnier and Didier Barbry*
Laboratoire d’Ingénierie Moléculaire, Université des Sciences et Techniques
de Lille, F-59655 Villeneuve d’Ascq (France)
Tel. / Fax +33 (0)320 434974,
E-mail: [email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Introduction
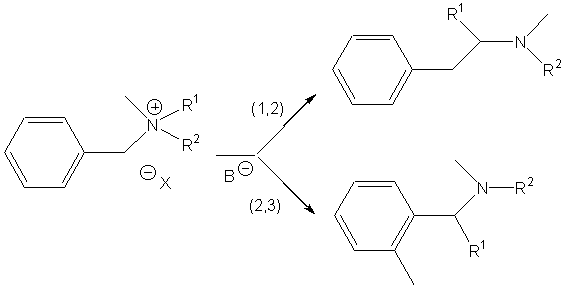
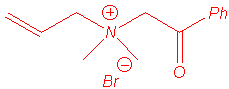
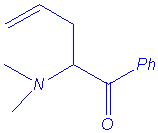
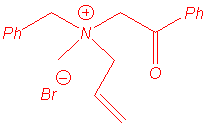
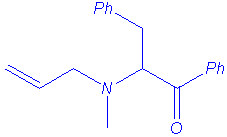
Ammonium ylides can be generated by treatment of ammonium salts with strong bases1, or with cesium fluoride (non-basic medium) when the ammonium bears a trimethylsilylmethyl group2. These ylides isomerise to (1,2) rearrangement products or to (2,3) shift products when allyl or benzyl substituents are located on the nitrogen atom (in some cases, (1,4) rearrangement has been also reported).

The Sommelet-Hauser rearrangement is a (2,3) sigmatropic shift whereas the Stevens rearrangement may follow one of three processes :

Scheme – Temperature rising (Table 1, entry 1) using microwaves and conventional heating
The rearrangement of ammonium ylides is especially related to the following parameters : steric hindrance of nitrogen substituents, temperature, medium polarity and strength of the base.
|
|
|
|
|
(Microwaves) % |
(Conventional heating) % | |
|
|
 |
|
 |
|
|
|
|
|
 |
|
 |
|
|
|
|
|
 |
|
 |
|
|
|
|
|
 |
|
 |
|
|
|
|
|
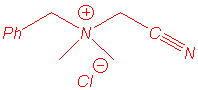 |
|
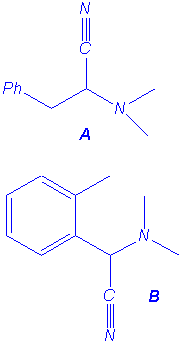 |
|
(A:B 70:30) |
(A:B 10:90) |
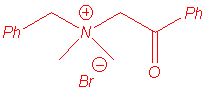
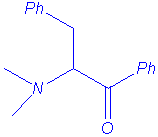
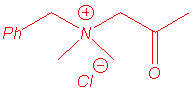
We isolated the same products under microwave irradiation and by classical heating. In particular, the benzyl group has a larger ability to migration than the allyl group and mixtures of products are obtained in the case of the entry 5.
However we have noticed a strong microwave effect: in all
cases the reactions are faster and microwave irradiation promotes the Stevens
reaction while conventional heating favours the (2,3) shift in
benzylcyanomethyldimethylammonium ylide. Studies are in progress to explain that
particular behaviour and to perform the tandem ammonium salt formation-ylide
rearrangement under microwave irradiation with a-aminonitriles4 and a-aminoesters5.