Microwave acceleration of the Pechmann reaction on graphite /montmorillonite K10: application to the preparation of 4-substituted 7-aminocoumarins
Stéphane Frère, Valérie Thiéry, Thierry Besson
Laboratoire de Génie Protéique et Cellulaire, EA3169, Groupe de Chimie Organique, UFR Sciences Fondamentales et Sciences pour l'Ingénieur, Université de la Rochelle, Avenue Michel Crépeau, F-17042 La Rochelle Cedex 1, France.
[email protected] [email protected] [email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Keywords: coumarins, Pechmann reaction, microwave irradiation.
Coumarins are very present in Nature and find their main applications as
fragances, pharmaceuticals and agrochemical.1 In the course of our
recent work on the preparation of new polyheterocyclic systems with potent
pharmacological value,2 we planned to prepare aminocoumarins which
can be employed as intermediates in the synthesis of useful bioactive
compounds.3 Such molecules are usually synthesised by a Pechmann
reaction which involve condensation of phenols with b
-ketonic esters in the presence of various reagents (e.g.
H2SO4). In some methods, mixtures of substituted phenols,
b -ketoesters and the acidic catalyst were allowed to
stand overnight or for a number of days (depending on their reactivity) or were
heated above 150°C, and undesired products, like chromones, in addition of
coumarins have also been reported. In the hope to perform convenient and
reproducible methods for the preparation of the rare amino compounds, we
transposed this Pechmann reaction to a focused microwave oven (open oven,
monomode system S402 Prolabo)4 especially designed for organic
synthesis. There are few papers on the use of microwave activation of Pechmann
reactions and all are reporting reactions which started from resorcinols or
activated alkoxyphenols5 but, none of them described the preparations
of aminocoumarins. In this paper we report an original and environmentally
friendly solvent free procedure and we studied the opportunity to use solid
supports and acid catalysts in such experiments.
Synthesis of aminocoumarin-4-carboxylic acid derivatives
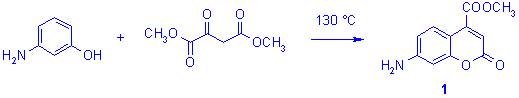
Synthesis of the methyl ester of 7-aminocoumarin-4-carboxylic acid 1 usually consists in the heating of the starting m-aminophenol with dimethyl oxalacetate, at 130 °C for 2h (scheme 1).6

Scheme 1
The amount of coumarins obtained in such conditions is variable and sometimes low (e.g. 36%, see Table 1), accompanied by complicated mixtures of carbonaceous compounds and coloured impurities which are very difficult to eliminate, even by column chromatography and recrystallization. Transposition of this procedure to a focused microwave reactor (130 °C, 60W) led to a reduction of the reaction time (85 min) in a similar yield (39 %). Because it is well known that the use of acid catalysts may play an important role in rate enhancement in Pechmann reaction,3 few drops of sulphuric acid were added to the starting mixture. Unfortunately, whatever conditions were used (thermal heating or microwave irradiation) no improvements of the reactions were observed, the yields became lower and a lot of by-products were also detected.
Table 1. Selected results on the
preparation of the
7-aminocoumarin 1.7,8a
________________________________________________________________________
Experimental conditionsa Conventional heatingb Microwave irradiation
Reaction time (min) Yield (%) Reaction time (min) Yield (%)
________________________________________________________________________
Neat (fusion) 120 36 85 39
Support: graphite 120 44 50 44
Support: graphite/K10 (2:1)c,d,e 66 64 30 66
________________________________________________________________________
(a) Reactions performed at 130°C; Lowest (110°C) or highest (150°C and 170°C) temperatures involved uncomplete or complex (degraded) reactions and poor yields of the attempted products.
(b) Oil bath.
(c) The ratio between the quantity of reactant and the graphite is very important; if it is too large or too small, degraded or incomplete reactions were observed.
(d) No modifications were observed when a preliminary activation (2h at 180°C) of the clay was realized.
(e) No significant results were observed in absence of graphite (montmorillonite K10 + phenol + b-ketoester)
Graphite is one of the solids most efficiently heated by microwaves and is also known for its adsorbing properties of organic molecules.9 Inspired by a recent work on Friedel-Crafts acylation reactions with carbon graphite as support,10 we showed that irradiation of a mixture of m-aminophenol and dimethyl oxalacetate (1 equiv.), adsorbed on graphite, led to the expected 7-aminocoumarin 1 in a yield of 44% and in a shorter time (50 min) than for the purely thermal procedures (see Table and Scheme 2) (in similar experimental conditions with same amount of starting materials and graphite, a conventional heating needed 120 min to give the expected product in similar yield). Here again addition of a catalytic amount of sulfuric acid led to a very complex reaction.
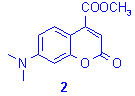
In order to avoid the use of mineral acids (e.g. H2SO4), we studied the opportunity to associate graphite to montmorillonite clay which have been used as efficient solid acidic catalyst for a variety of organic reactions.11 Then, treatment of m-aminophenol with the tertiary b -ketonic ester was carried out on a mixture of graphite/montmorillonite K10 under microwave irradiation. Various parameters were studied and optimized (reaction time: 30 min, temperature: 130°C, and ratio of graphite/montmorillonite Õ 2:1 w/w) and we also found that the optimum amount of solid support was 75% by weight of the global reactants (Table 1). Extension of this procedure to various aminophenols was performed and results obtained (Table 2) are in accordance with data observed for the synthesis of coumarin 1.12
Table 2. Synthesis of 7-aminocoumarins
derivatives 2 and 3.7,8a
|
|
|
||
|
|
Reaction time (min) Yield % | Reaction time (min) Yield % |
|
 |
|
|
|
 |
56 68 |
8 75 |
156-158b |
(a) Reactions performed at 130°C. Support:
graphite/K10 (2:1); (b) mp lit.13: 156 °C.
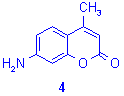
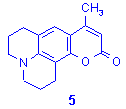
Synthesis of 7-amino-4-methylcoumarins.7,8b
In this case the starting b-ketonic ester (ethyl acetoacetate, CH3COCH2COOC2H5) is a liquid. Mixing aminophenols14 and ethyl acetoacetate on graphite/K10 (2:1, w/w) led to an heterogeneous mixture and resulted in hazardous bumping in the reactor and uncomplete reactions with a large amount of by-products (only 5% yield of attempted compound). The best alternative consisted into the microwave exposition of phenols and ethyl acetoacetate in the presence of concentrated sulphuric acid. Here again, the comparative study of this procedure by classical heating and microwave irradiation showed that reaction time was reduced from several hours to only a few minutes by using the latest technique. On this point these experiments are showing the difficulties to associate liquid and solid phase in microwave reactions, but it confirms that, in favourable conditions, focused microwave irradiation is a very powerful technique for accelerating thermal organic reactions.
Table 3. Synthesis of 7-aminocoumarins
derivatives 4 and 5.7,8b
|
|
|
||
|
|
Reaction time (min) Yield % | Reaction time (min) Yield % |
|
 |
|
|
|
 |
390 62 |
12 62 |
94-96 |
(a) Reactions performed at 130°C. Support: graphite/K10 (2:1); (b) mp lit.15: 220-224 °C.
Conclusion
In conclusion, we performed the synthesis of rare aminocoumarins via Pechmann reaction by exposition of the reactants to a microwave field, on original support (graphite/montmorillonite K10). In this efficient new methodology, the strong thermal effect due to graphite/microwaves interaction is associated to the acidic catalyst role of the clay.
In relation with our recent published results,16 this work also demonstrated that working under focused microwave irradiation need a special attention: a) the ratio between the quantity of the material and the support (or the solvent)16 is very important, b) for solid starting materials, the use of solid supports offer operational, economical and environmental benefits over conventional methods. In contrast, association of liquid/solid reactants on solid supports may involved uncontrolled reactions and are generally worse than comparative thermal reactions. In this case, simple fusion of the products or addition of an appropriate solvent may lead to more convenient mixtures or solutions for microwave applications.
Acknowledgements
We thank the Comité de Charente-Maritime de la Ligue Nationale contre le Cancer and the Communauté d'Agglomération de La Rochelle (S.F. PhD grant) for financial support.
References and notes
2. (a) Bénéteau, V.; Besson, T.; Guillard, J.; Léonce, S.; Pfeiffer, B. Eur. J. Med. Chem. 1999, 34, 1053-1060; (b) Bénéteau, V.; Pierré, A.; Pfeiffer, B.; Renard, P.; Besson, T. Bioorg. Med. Chem. Lett. 2000, 10, 2231-2234; (c) Lamazzi, C.; Leonce, S.; Pfeiffer, B.; Renard, P. ; Guillaumet, G.; Rees, C. W. Bioorg. Med. Chem. Lett. 2000, 10, 2183-2185.
3. Sethna, S.; Phadke, R. Org. React. (N. Y.) 1953, 7, 1-58.
4. Commarmot, R.; Didenot, R.; Gardais, J. F. French Patent 84/03496, 1986; Chem. Abst. 1986, 105, 17442.
Focused microwave irradiations were carried out at atmospheric pressure with a Synthewave S402 Prolabo microwave reactor (300 W, monomode system) which has quartz reactors, visual control, irradiation monitored by PC computer, infrared measurement and continuous feedback temperature control (by PC). Equipment of the oven can be completed by an external stirring system, a condenser and dropping funnel allowing conditions close to those involved in classical methods; it is also possible to work under dry atmosphere or in vacuo if necessary.
5. (a) de la Hoz, A.; Moreno, A.; Vasquez, E. Synlett 1999, 608-610; (b) Singh, J.; Kaur, J.; Nayyar, S.; Kad, G. L. J. Chem. Res. (S) 1998, 280-281; (c) Li, T. S.; Zhang, Z. H.; Yang, F.; Fu, C. G. J. Chem. Res. (S) 1998, 38-39.
6. Kanaoka, Y.; Kobayashi, A.; Sato, E.; Nakayama, H.; Ueno, T.; Muno, D.; Sekine, T. Chem. Pharm. Bull. 1984, 32, 3926-3933.
7. All compounds were fully characterised by spectroscopy and elemental analysis.
8. a) Typical procedure for the synthesis of compounds 1-3: A mixture of the aminophenol (2 mmol), dimethyl oxalacetate (2 mmol) and graphite/montmorillonite K10 (2:1, w/w) (75wt% to the phenol and b-ketoester) were placed in a quartz vial (250 ml) inside the oven and irradiated for 30 min. The irradiation was programmed to obtain a constant temperature (130°C) with a maximal power output of 90W. After cooling, the mixture was filtered (methanol) and the crude product was purified by column chromatography (silica gel) with light petroleum-dichloromethane as the eluent.
b) Typical procedure for the synthesis of compounds 4-5: A mixture of aminophenol (2 mmol) and ethyl acetoacetate (2mmol) was placed in a quartz vial (70 ml) and conc. H2SO4 (3 ml) was added. The mixture was then subjected to microwave irradiation, at 130°C, for an optimized time (8 min) with a maximal power output of 90 W. After completion of the reaction (monitored by TLC), dichloromethane was added, the organic layer washed with water, dried over MgSO4, and the crude product was purified as above.
9. Walkiewicz J. W.; Kazonich, G.; Mc Gill, S. L.; Min. Metall. Process. 1988, 5, 39-42.
10. (a) Laporte, C.; Baulès, P.; Laporterie, A.; Desmurs, R.; Dubac, J. C. R. Acad. Sci. Paris 1998, t.1, Série IIc, 141-150; (b) Laporte, C.; Marquié, J.; Laporterie, A.; Desmurs, R.; Dubac, J. C. R. Acad. Sci. Paris 1998, t.2, Série IIc, 455-465.
11. For a complete review see: Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathé, D. Synthesis 1998, 1213-1234.
12. It must be noted that the lowest reaction times observed for the substituted starting amines are in accordance with previous results showing that alkylated aminophenols were more reactive than their free analogs. (See ref. 3).
13. Besson, T.; Coudert, G.; Guillaumet, G. J. Heterocycl. Chem. 1991, 28, 1517-1523.
14. In this case the starting m-aminophenol had to be protected in ethyl carbamate.
15. Atkins, R.; Bliss, D. J. Org. Chem. 1978, 43, 1975-1980.
16. (a) Besson, T.; Guillard, J.; Rees, C. W. Tetrahedron Lett. 2000, 41, 1027-1030; (b) Soukri, M.; Guillaumet, G.; Besson, T.; Aziane, D.; Aadil, M.; Essassi, El M.; Akssira M. Tetrahedron Lett. 2000, 41, 5857-5860; (c) Besson, T.; Guillard, J. Tetrahedron 1999, 55, 5139-5144; (d) Besson, T.; Dozias, M. J.; Guillard, J.; Jacquault, P.; Legoy, M. D.; Rees, C. W. Tetrahedron 1998, 54, 6475-6484.