[A010]
NEW ORGANIC MATERIALS: THE SYNTHESIS OF RADİCAL CATİON SALTS OF THIOMETHYLNAPHTHALENE
Nurgün Büyükkıdan*, Ayşegül Öncü, Bulent Büyükkıdan and Osman Çakmak
Department of Chemistry, University of Gaziosmanpasa, 60250, Tokat, Turkey
FAX: +90 356 2521585; E-mail: [email protected]
ABSTRACT
Charge transfer complex, which are existed from organic π-electron donor and electron acceptor molecules call attention. The halogenation of pyrene leads in quantitative yield to 1,3,6,8-tetrahalopyrene which reacts with sodium methyl sulfide in aprotic solvents to tetrakis(methylthio)pyrene with optimal yields
of 98%. Electrocrystallization of yield in the presence of suitable electrolytes gives the radical cation salts with powder conductivities between 10-1 and 10-2 S.cm-1. This compound shows single crystal conductivities of 300 to 677 S.cm-1 which increase with decreasing temprature (1). The same results have been observed in the 1,8 peri-bridge compounds of naphthalene.
INTRODUCTION
Perylens conductivity is rather low value (1х10-15 S-1). Despite this, the conductivity of perylenssalt with brom is rather high(10-1 S-1). This difference has shown that molecule materials can also show high electrical conductivity. This observation is the start of the studies inthis field. In later yields, powerfull acceptor 7,7,8,8-tetracyano-quinodimethane(TCNQ) has been synthesed and lots of salt which shows electrical conductivity, has been obtained.
Different from this, BEDT-TTF charge transfer complex which Urayama has been synthesed, shows high conductivity(Schame-1). But, the disadvantage of this kind of reactions is the difficulty of the synthesis of donor molecules and the low stability of radical cation salts.

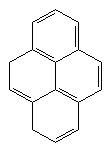
BEDT-TTF Perilen
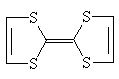
TTF
Schame-1
In last years it is seen that sulphur, selenium and tellurium derivatives of aromatic compounds and radical cation salt has electrical conductivity. So, the studies in this field have icreased. Although the radical cation salts of simple arenes such as naphthalene, anthrasene, pyrene and perylene show electrical conductivity (10-1-10-2 S.cm-1), they are rather unstable. But, when sulphur is bound to such kind of arenes, the stability of radical cation rather increases.
In 1980, Bechgaard and his group register great development on molecular metals field and synthesed in series (TMTSF)2X salts(X=PF6-, AsF6-, NO3- or CIO4-) which showed high conductivity with electrocrystallization method(Scheme-2).
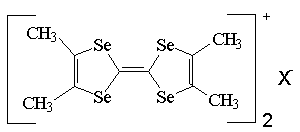
Tetramethyltetraselenofulvalen (TMTSF)2X
Schame-2
For the synthesis of these sulphur derivatives first, dibrom derivatives has been synthesed. In the second step, peri-bridged chalcogen compounds are formed as
a result of the treatment with elemental sulphur and organo-metal dialkillithium salt which is obtained with the reaction of metal-halogen exchange(Schame-3).

Schame-3
Meinwald and his group(1977) have synthesed asimetric bridged sulphur-selenium-tellurium naphthalene derivatives. Charge transfer complex and radical cation salts of this donor naphthalenes which are formed withTCNQ and iodine, have been showed electrical conductivity(Schame-4).
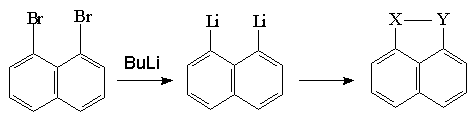
X= S , Y= Se
X= S , Y= Te
X=Se , Y= Te
Schame-4
As perisulphur bridged mololecular conductors, benzanthresen and anthrasen donor molecules are also synthesed(Schame-5). Not only peri-bridged sulphur arenas, but also alkhil sulphur derivatives have shown activity and function in the same way.
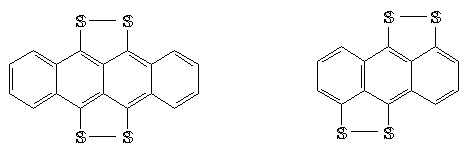
Schame-5
As seen, methylsulphur arens as peri-bridged sulphur derivatives, can be easily synthesed from their bromo derivatives and radical cation salts show high conductivity.
RESULT AND DISCUSSION
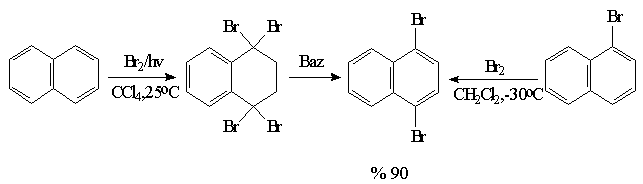
In this study, the synthesis of methylsulphur derivatives is aimed at first step. For this, bromonaphthalenes will be treated with the reactives like n-BuLi or t-BuLi , then they will be turned into methylsulphur derivatives with the
electrophils like CH3SSCH3. The synthesis of 1,4 and 1,5 bis(thiomethyl)naphthalene is showed below as an example(Schame-6).
Synthesis of 1,4-bis(thiomethyl)naphthalene:
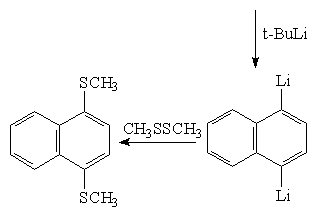
Synthesis of 1,3,5-tris(thiomethyl)naphthalene:
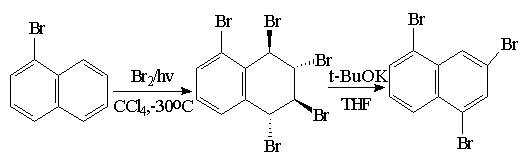

Schame-6
In the second step of the study, electrocrystallization method will be applied to form radical cation salts of the methylsulphur derivatives naphthalene (Schame-7). The electrical conductivity of PF6-, BF4-, CIO4- and I- salts which are obtained by this method will be measured.
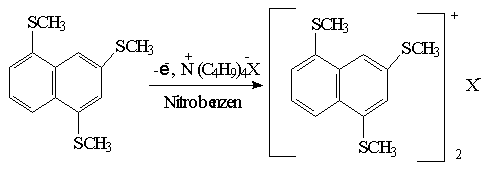
Schame-7
In contrast to polymers, the major disadvantage of molecular metals is that there is very limited tenability: only unique combinations and stoichiometric ratios of donors and acceptors yield conductive materials. The key is stacking of the donors or acceptors in a manner allowing for band formation and thus partial charge-transfer. This is a delicate function of the compoundsshape and size, Madelung forces and crystallisation conditions. This stacking requirement further leads to low-dimensionality in this molecular metal-like materials. However, the most successful, superconducting molecular conductors often have somewhat higher dimensionality to allow conduction between parallel chains.
It has imparted greater stability to the radical cation salts, coupled with increased conductivity, and some systems, notably salts of tetrathiotetracene retain metallic conductivity to very low temperatures.
CONCLUSION
Our studies are going on with the charge-transfer complex of donor methyl sulphur naphthalene which we synthesised, the existence of radical cation salts and their measurement of electrical conductivity.
REFERANCES
CHIANG, I., and MEINWALD, J., 1980. Peri-Bridged Naphthalenes. 4. Chalcogen-Bridged Acenaphthylenes, Tetrahedron Letters, Vol. 21, 4565-4568.
ÇAKMAK, O., 1999. Bromination of Naphthalene, Preperation of 1,3-Dibromonaphthaline. J. Chemical Research (S), 366-367.
ÇAKMAK, O., KAHVECİ, İ., DEMİRTAŞ, İ., HÖKELEK, T., and SMITH, K., 2000. Bromination of Tetralin. Short and Efficient Synthesis of 1,4-Dibromonaphthalene, Collect. Czech. Chem. Commun., Vol. 65
ÇAKMAK, O., DEMİRTAŞ, İ., and BALAYDIN, H., T., 2002, Tetrahedron, 58, 5603-5609
DESMURS, j. R., 1993. Advances in Organobromin Chemistry II, 72-73.
HEYWANG, G., and ROTH, S., 1991. Radical Cation Salts of 1,3,6,8-Tetrakis-(methylthio)Pyrene-New Easily Accessible Compounds with High Electrical Conductivity and Excellent Stability, Angew. Chem. Int. Ed. Engl., 30, 176-177.
MEINWALD, J., and DAUPLAISE, D., 1977. Peri-Bridged Naphthalenes. 2. Unsymetrical Diatomic Chalcogen Bridges, J. Am. Chem. Soc., 99, 7743-7744.
UNDERHILL, A. E., 1992. Molecular Metals and Superconductors, J. Mater. Chem., 2, I-II.
URAYAMA, H., YAMOCHI, H., SAITO, G., NOZAWA, K., SUGANO, T., KINOSHITA, M., SATO, S., OSHIMA, K., KAWAMOTO, A., TANAKA, J., 1988. Chem Lett., 55.