[A013]
Synthesis of benzodifuran
derivatives by using 2-aryl-1,4-benzoquinones
Vasyl S. Matiychuk, Roman L. Martyak, Mykola D.
Obushak*
Department of Organic Chemistry,
Kyryla & Mefodiya 6,
E-mail: [email protected]
Abstract:
2-Aryl-1,4-benzoquinones (aryl = RC6H4,
R = H, 4-Me, 3-CF3, 4-COOH, 3-Cl, 4-Cl, 4-F, 4-NO2) react
with methyl ethyl, propyl and allyl cyanoacetates in
the presence of some bases to form dialkyl
2,6-diamino-4-arylfuro[2',3':4,5]benzo[b]furan-3,7-dicarboxylates. Regardless
of the reagents ratio benzodifuran derivatives are formed selectively. Only in
reaction 2-(4-nitrophenyl)-1,4-benzoquinone with ethyl
cyanoacetate 2-amino-5-hydroxy-4-(4-nitrophenyl)benzo[b]furan-3-carboxylate is
formed as minor component.
Key words: benzoquinone, 2-aryl-1,4-benzoquinones,
cyclizations, heterocycles, benzodifuran derivatives, cyanoacetic
esters, arylation, Meerwein reaction.
It is known that interaction of quinones with
various C-nucleophiles is often not finished by 1,4-addition.
In the presence of other functional groups intramolecular cyclization takes
place to form condensed heterocyclic compounds [1]. However, mainly 1,4-benzoquinone
and also disubstituted quinones were investigated in these reactions. Due to
asymmetry of monosubstituted 1,4-benzoquinones a
formation of different isomers is possible in the reactions with
C-nucleophiles. Therefore, such quinones are studied considerably less in these
reactions, but in published works [1–7] some contradictions which concerned
their regioselectivity took place. It was reported that toluquinone
and 2-chloro-1,4-benzoquinone react with ethyl
acetoacetate or ethyl benzoylacetate to form linear
furo[2',3':4,5]benzo[b]furan isomers [2, 3]. Under changed the reaction
conditions benzofuran derivatives (1:1 adduct) was received, moreover CH-acid
added in position 5 of 2-aryl-1,4-benzoquinones
[2]. Subsequently
evidences were found that 1:1 adduct formed as a result of nucleophilic attack
in position 6 [4]. The condensation of ethyl benzoylacetate with toluquinone
has been reported to lead to two benzofuran isomers [4, 5]. However, for one of
them in these works a different structure was assigned. The ratio 1:1 and 1:2
adducts largely depends on reaction conditions [6, 7]. From result obtained at
analysis of interaction of 2-acetyl-1,4-benzoquinone with CH-acid is possible
to make the conclusion that the electron-withdrawing groups in quinone ring direct nucleophilic attack to
position 3 [8–10]. Unsubstituted quinone react mainly with cyanoacetic esters
to form dialkyl 2,6-diaminofuro[2',3':4,5]benzo[b]furan-3,7-dicarboxylate
[11, 12]. In this report interaction
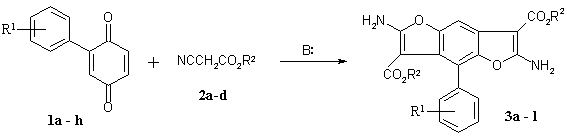
of 2-aryl-1,4-benzoquinones 1a–h with cyanoacetic esters 2a-d is investigated. Compounds 1a–h are prepared by arylation of the 1,4-benzoquinone with arenediazonium chlorides [13–15].
In similar reactions
quinone derivatives may react with one or two molecules of CH-acid by the type
of Michael reaction [16–19] with followed cyclization to derivatives of
benzofuran and benzodifuran respectively. We have established that quinones 1a–h react with cyanoacetic esters 2a-d to form benzodifuran derivatives 3a–l (Scheme 1).

1: R1=H (a), 4-Me (b), 3-CF3 (c),
4-COOH (d), 3-Cl (e), 4-Cl (f), 4-F (g), 4-NO2
(h);
2: R2=Me (a),
Et (b), Pr (c), Allyl (d).
|
3 |
R1 |
R2 |
Yield, % |
|
a |
H |
Me |
50 |
b
|
3-CF3 |
Me |
42 |
|
c |
4-F |
Me |
54 |
|
d |
H |
Me |
49 |
|
e |
4-Me |
Et |
56 |
|
f |
4-COOH |
Et |
46 |
|
g |
3-Cl |
Et |
44 |
|
h |
4-NO2 |
Et |
31 |
|
i |
H |
Pr |
34 |
|
j |
4-F |
Pr |
38 |
|
k |
H |
Allyl |
58 |
|
l |
4-Cl |
Allyl |
62 |
Reaction was carried out in alcohol in the
presence of bases (ammonium hydroxide, piperidine, alcoholates).
Arylquinones with ortho-substituents in aromatic ring were practically
unreactive in this reaction. In those cases benzodifuran or benzofuran
derivatives are not isolated in pure form.
The interesting particularity of the reaction
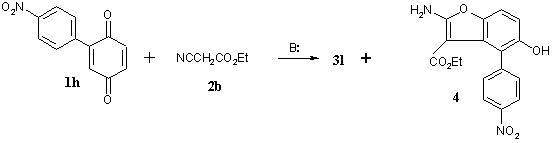
is that regardless of the reagents ratio benzodifuran derivatives are formed selectively. Only in the reaction of ethyl
cyanoacetate 2b with 2-(4-nitrophenyl)-1,4-benzoquinone 1h minor
component – ethyl
2-amino-5-hydroxy-4-(4-nitrophenyl)benzo[b]furan-3-carboxylate 4 (Scheme
2) – is formed besides of benzodifuran 3h (3h:4 =
77:23).
Probable mechanism of this reaction includes
several stages (Scheme 3). Carbanion of cyanoacetic ester reacts with quinones 1a–h
by the type of Michael reaction with the formation of hydroquinone A.
The latter is oxidized by starting quinone to substituted quinone B. In
the next stage another molecule of cyanoacetic ester is added to quinone B.
Finally intramolecular cyclization takes place due to interaction of hydroxy
and nitrile groups of hydroquinone C.
The principal stage of this process evidently
is fast oxidation of adduct A to substituted quinone B. In that
case when primary addition product A had not time to oxidize, its
intramolecular cyclization takes place to form benzofuran derivative 4.
Electron-withdrawing substituents in aromatic ring of compounds 1a–h (R1=NO2)
naturally favour to such reaction route.

In 1H NMR spectra of compounds 3a–l
two sets of signals of ester groups protons are
observed. It is explained by shielding of one of ester groups by aryl
substituent. Indeed in 1H NMR spectrum of diethyl 2,6-diamino-4-methylfuro[2',3':4,5]benzo[b]furan-3,7-dicarboxylate
5, was obtained by interaction of ethyl cyanoacetate with toluquinone (Scheme 4), signals of CO2Et-groups protons practically
coincide.

Scheme 4
So, during the interaction of arylquinones with
cyanoacetic esters the reaction is not stoped on the stage of the addition of
one molecule of nucleophilic reagent. Adduct 1:2 is formed and its
intramolecular cyclization leads to benzodifuran derivatives.
6.
Kornilov M.Yu., Makovetskii V.P., Turov A.V. and Dzvinchuk I.B. Khim. Geterotsikl. Soedin. (Chem.
Heterocycl. Compds) 1980, 22.
13.
Obushak M.D., Matiychuk V.S.
and Martyak R.L. Khim. Geterotsikl. Soedin. (Chem. Heterocycl.
Compds) 2001, 986.
14. Brassard P. and L'Écuyer P. Can. J. Chem. 1958, 36,
700.
15. Kvalnes D.E. J. Am. Chem. Soc. 1934, 56, 2478.
19.
Makovetskii V.P., Dzvinchuk I.B.,
Volovenko Yu.M. and Svishchuk À.À. Khim. Geterotsikl. Soedin. (Chem.
Heterocycl. Compds) 1979, 129.