Sixth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-6), 1-30 September
[A014]
Synthesis of Iodylarenes from Iodoarenes, with Sodium Periodate as the Oxidant. Part II.
Lukasz Kraszkiewicz and Lech Skulski*
Chair and Laboratory of
Organic Chemistry, Faculty of Pharmacy, Medical University,
PL 02-097
Warsaw, Poland, 1 Banacha Street
Tel/fax:+48(22)5720643; E-mail: [email protected]
Abstract: An improved method is reported for the effective preparation of some iodylarenes, ArIO2, from the corresponding iodoarenes, ArI, using sodium periodate, NaIO4, as the oxidant dissolved in boiling 30% aq. CH3COOH solutions. The former reaction times, given in Part I, were shortened from 8-16 hours to 3-6 hours, with preserving the same good yields and high purities of the crude final products, ArIO2, i.e. 96-99%.
Keywords: iodylarenes, iodoarenes, sodium periodate, oxidation
During the past 20 years, iodylarenes,
ArIO2, the aromatic compounds of iodine(V), have been widely
used in organic synthesis as mild and very selective oxidants. The most
important iodylarenes are: iodylbenzene, 4-(t-butyl)iodylbenzene,
"2-iodylbenzoic acid"
[i.e. 1-hydroxy-1,2-benziodoxol-3-(1H)-one
1-oxide] and particularly its derivative, named the Dess-Martin reagent
[1,1,1-triacetoxy-1,2-benziodoxol-3-(1H)-one] [1].
In Part I [2], we reported a new method for preparing iodylarenes from iodoarenes, in the boiling and vigorously stirred neutral aqueous sodium periodate solutions. Those reactions proceeded within 8-16 h and gave ArIO2 in 58-91% crude yields, having a 97-99% purity established iodometrically.
Recently, in our labolatory we worked up an
improved method for preparing ArIO2 from ArI. We
observed that when the above boiling reaction mixtures were acidified with
dilute H2SO4, the yellowish iodosylarenes, ArIO,
were the main products of the reactions. When we used varied boiling aqueous
AcOH solutions, the initially precipitated out yellowish ArIO, after 0.5-1.5 h
were transformed into colorless ArIO2. According to these
observations, we have already suggested that the reactions undergo in two steps:
ArI are oxidized to ArIO, and the latter ones are readily
diproportionated to give ArI and ArIO2 [2]. We have
established that the optimum concentration of AcOH aqueous solutions for
preparing ArIO2 is just 30%. Using this binary acidic
solvent, we considerably shortened the reaction times and, in some cases, we
increased the yields. Iodometric titrations [3] showed a 96-99% purity of
the obtained crude iodylarenes. However, 3-iodobenzoic acid gave a mixture of
3-iodosyl and
3-iodylbenzoic acids; cf. Part I.
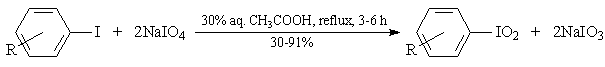
R - see Table 1
Improved Procedures for Preparing Iodylarenes from Iodoarenes:
NaIO4 (4.70 g, 22
mmol, 10% excess) was suspended in 30% (v:v) aqueous AcOH solution. An
appropriate iodoarene (10 mmol) was added (for steam-volatile ArI, a few
drops of toluene were also added; see Part I for the explanation). The
mixtures were vigorously stirred and refluxed for 2-6 h. In some cases, when the
mixtures had still yellowish color after this time, 20 ml of boiling water was
added, and the resulting mixtures were further refluxed for the next 1-1.5 h
(see Table 1). After an appropriate time, the temperature was lowered to r.t.
The crude products were collected by filtration, washed on the filter with ice
cold water (3 x 10 mL) and acetone (3 x 10 mL) and they were air-dried in the
dark.
As previously [2], small samples of the crude ArIO2
were recrystallized from boiling water, in order to obtain the analytical
specimens.
Table 1. The reaction times, crude yields, and melting points (uncorrected) for the recrystallized ArIO2.
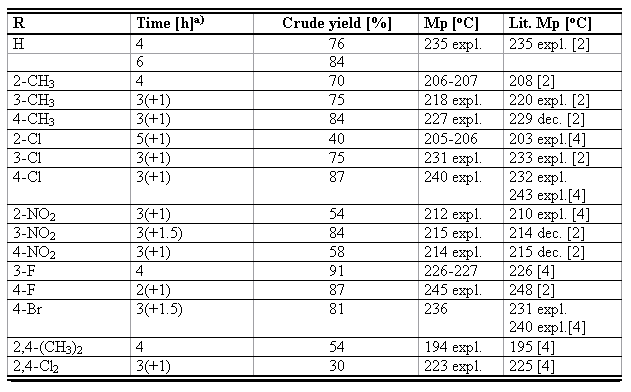
a) The reaction time after adding the additional amount of boiling water (20 mL) is given in parentheses.
References
1. Zhdankin, V. V.; Stang, P. J.; Chem. Rev. 1996, 96, 1123.
2. Kazmierczak, P.; Skulski, L.; Kraszkiewicz, L. Molecules 2001, 6, 881.
Avail. at URL: http://www.mdpi.org/molecules/papers/61100881.pdf
3. Lucas, H. J.; Kennedy, E. R.; Formo, M. W. Org. Synth. 1942, 22, 70.
4. Beriner, F. M.; Gindler, E. M. Iodine Abstr. Rev. 1956, 3, 1.