
[B001]
A Panoply of Polymer-Anchored Cinchona Alkaloids for Asymmetric Phase-Transfer Catalysis
Baptiste Thierry, David Paramelle, Jean-Christophe Plaquevent, and Dominique Cahard*
Université de Rouen, UMR 6014 de l'Institut de Recherche en Chimie Organique Fine (IRCOF), Rue Tesnière 76821 Mont Saint Aignan Cedex, France
E-mail: [email protected]
Abstract: Design, synthesis and application of unprecedented polymer-supported cinchona alkaloid derivatives as phase-transfer catalysts are reported in this communication. Both soluble and insoluble structures were studied. We have demonstrated that the benzylation of glycine Schiff base derivatives can be achieved in up to 94% ee.
Keywords: a -amino acids, polymer-supported catalysts, asymmetric phase-transfer catalysis, cinchona alkaloids
The asymmetric synthesis of a -amino acids remains a major challenge in organic chemistry.1 An attractive method, first introduced by O’Donnell in 1989,2 used the liquid/liquid phase-transfer catalysed asymmetric alkylation of N-diphenyl methylene glycine t-butyl ester (scheme 1) with the aid of N-benzyl cinchona alkaloid salts as phase-transfer catalysts (first generation, see figure 1).

Scheme 1 : Enantioselective benzylation of N-diphenyl methylene glycine t-butyl ester.

Figure 1: The generations of cinchona alkaloid phase-transfer catalysts.
Two further generations of catalysts derived from cinchona alkaloids were subsequently developed. The second generation of catalysts, i.e. the N-alkyl O-alkyl cinchona alkaloid salts were reported in 1994,3 also by O'Donnell and co-workers. Finally, the third generation of catalysts was described independently by Lygo4 and Corey5 in 1997 in which a 9-anthracenylmethyl group was introduced as an effective unit for masking the nitrogen face, leading to substantially improved enantiomeric excesses (figure 1).
During the last two years, our contribution in this field consisted of the anchorage of the cinchona alkaloid on a polymeric support to produce unprecedented polymer-supported phase-transfer catalysts. Such catalysts present several advantages: simplified work-up for product purification, easy recovery and potentially recycling, stability, less toxicity.
Few examples of polymer-supported phase-transfer catalysts (PS-PTC) have been reported in the literature.6 When we initiated the project, the best reported result on enantioselective alkylation of N-diphenyl methylene glycine t-butyl ester was described by Zhang, leading to 27% ee, using cinchona alkaloids grafted on Merrifield resin at the quinuclidinium nitrogen atom (figure 2). 7 Then, Najera et al reported improved e.e.s of up to 58%, 8 using the same supported cinchona alkaloids on benzylation of N-diphenyl methylene glycine t-butyl ester (figure 2), and an ee of 90% on benzylation of the i-propyl derivative.
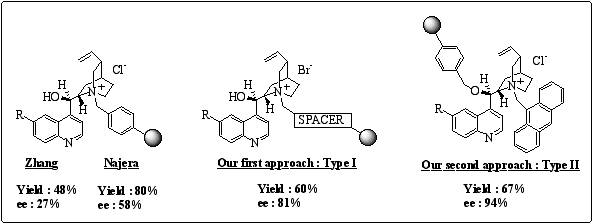
Figure 2
: Cinchona alkaloid supported phase-transfer catalysts in the benzylation of N-diphenyl methylene glycine t-butyl ester.We introduced new polymer-supported chiral phase-transfer catalysts possessing a spacer between the matrix and the quaternary nitrogen atom of the cinchona alkaloid (figure 2, Type I), leading to e.e.s up to 81%. 9 Worthy of note, the pseudoenantiomeric effect was not observed with those catalysts, and recycling gave dramatic drop of the enantioselectivity.
In a second approach, we thought that enantioselectivity could be possibly improved by anchoring the alkaloid elsewhere than on the nitrogen. The hydroxyl function was selected for this purpose, leaving the nitrogen of the quinuclidine moiety free to be quaternarized with the aid of 9-(chloromethyl)-anthracene. With this type II polymer-supported catalyst, we improved e.e.s to up to 94%. 10 Besides, recycling this catalyst showed a moderate decreased of e.e from 94% to 74% in five consecutive runs.
We herein present the synthesis of a panoply of new polymer-anchored cinchona alkaloids and their application as asymmetric phase-transfer catalysts. In various applications (Michael addition, asymmetric dihydroxylation…) but not for phase-transfer catalysis, cinchona alkaloids were grafted on a polymeric matrix most commonly through the vinyl group and at the 9-hydroxy group. Cleavage of the methoxy group on the quinoleine moiety in order to release the hydroxyl function provides an interesting binding site, but it has never been reported in the literature (figure 3).
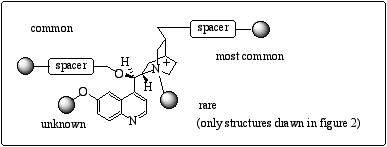
Figure 3
: Binding sites for cinchona alkaloids.We have prepared a panoply of polymer-anchored cinchona alkaloids using different strategies (figure 4). Both insoluble and soluble polymers have been considered. In particular, soluble polymers represent an interesting alternative since homogeneous reaction can be performed and the polymer can be readily separated by precipitation and filtration at the end of the reaction.
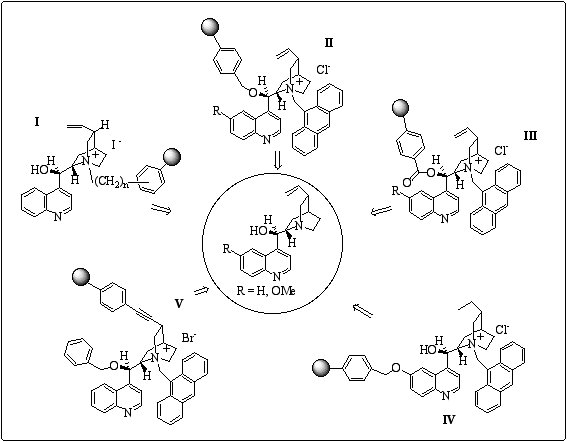
Figure 4
: A panoply of polymer-anchored cinchona alkaloids.Preliminary results of enantioselective induction are reported for each new supported catalyst whereas optimisation studies are in progress.
1-Type II catalysts
1-1 TentaGel resin
TentaGel HL-Br is an O - (2-bromoethyl) polyethylene glycol grafted onto a polystyrene core. The combinaison of non-polar polystyrene and polar polyethylene glycol results in resins that swell in a wide variety of solvents, including solvents that would shrink plain polystyrene-based resins. As a result, we expected a different behaviour for the cinchona ammonium salts that might lead to improved enantioselectivity in the alkylation of glycine Schiff base derivatives. The synthesis is presented in scheme 2.
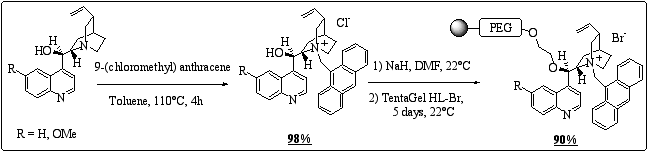
Scheme 2: Preparation of TentaGel-supported cinchona alkaloids.
We then evaluated the enantioselectivity induced by such polymer. The benzylation of N-diphenyl methylene glycine t-butyl ester was performed at 0 °C in a L/L/S system using aqueous KOH, toluene and the polymer-supported catalyst. To date, the best result, which was obtained, did not exceed 27% ee with the cinchonidinium salt.
1-2 Soluble Merrifield resin
We decided to synthesise a soluble analogue of Merrifield resin by polymerisation of 4-vinyl benzyl chloride in the presence of AIBN. The resulting resin was used to support the N-9 methyl anthracenyl cinchona alkaloids. (scheme 3) They were found to be soluble in toluene and in dichloromethane which are solvents used to run the enantioselective alkylation and insoluble in diethyl ether, thus rendering the recovery of the catalyst possible.
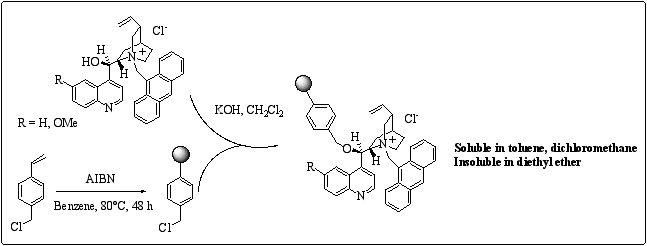
Scheme 3
: Preparation of soluble polymer-supported type II cinchona alkaloids.The best result that we obtained on benzylation of glycine Schiff base rise up to 85% ee, whereas in the same conditions, with insoluble polymer, results did not exceed 79% ee. Further studied are now in progress.
2- Type III catalysts
Having studied polymers bearing an ether linkage between the matrix and the alkaloid, we now considered grafting of the alkaloid through an ester function, which has an electro withdrawing behaviour.
2-1 Wang polymer
We were inspired by recent work by Lectka et al who described a polymer-supported quinine derivative applied to asymmetric catalysis on sequentially-linked columns.11 The polymer was prepared by including a terephtaloyl spacer between Wang resin and the cinchona alkaloid. In a similar approach, we prepared supported cinchona alkaloid salts in a one-pot procedure by successive addition of the Wang resin to the terephtaloyl chloride and then the N-9 methyl anthracenyl cinchona alkaloid to end up with the desired structure. (scheme 4)
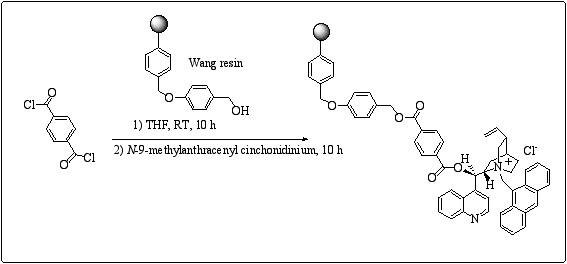
Scheme 4
: Preparation of Wang derived polymer-supported type III cinchona alkaloids.Interestingly, in a single attempt, we recorded an 82% ee in the benzylation of glycine Schiff base.
2-2 Insoluble and soluble carboxy polystyrene derivatives
The preparation of the insoluble polymer employs the commercially available carboxy polystyrene, which is transformed into the acyl chloride prior to react with N-9-methylanthracenyl cinchonidinium. (scheme 5) In 2-1, the benzyloxyterephtaloyl moiety represents quite a long spacer between the polystyrene core and the chiral ammonium, we then investigated the impact of the removal of the spacer on the catalyst behaviour in enantioselective alkylation.
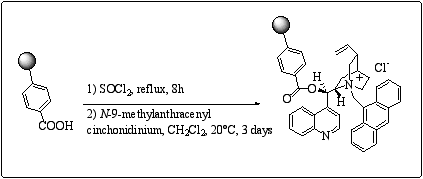
Scheme 5
: Preparation of carboxy polystyrene derived polymer-supported type III cinchona alkaloids.This kind of polymer-supported catalyst gave an ee of 30% in the benzylation of glycine Schiff base. In order to get soluble analogues of this type of polymer, the esterification of the cinchona alkaloids was performed with 4-vinyl benzoyl chloride. The soluble approach was inspired from Song's work who reported the preparation of poly (dihydro cinchona alkaloids 4-vinylbenzoate) applied to asymmetric dihydroxylation of olefins.12 Our synthesis follows Song's procedure, the quaternarization being carried out in the last step. (scheme 6)
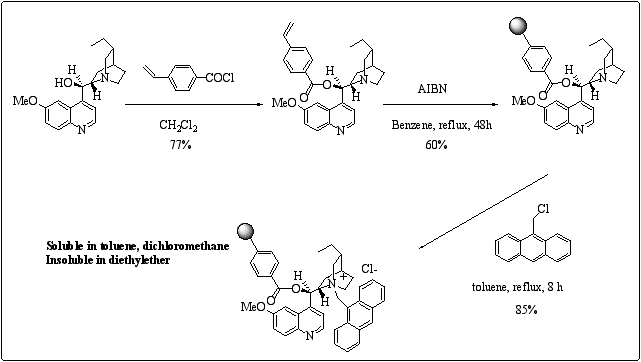
Scheme 6
: Preparation of polymer-supported soluble type III cinchona alkaloids.This soluble polymer gave an ee of 43% in the benzylation of glycine Schiff base, sensibly better than with the insoluble polymer. Further improvements of the enantioselectivity are in progress.
3- Type IV catalysts
Very few examples of demethoxylated quinine or quinidine derivatives are described in the literature.13 Moreover, there is no report concerning the anchorage of cinchona alkaloids onto the methoxy function. Demethoxylation of dihydroquini(di)ne was carried out following Heidelberger's method14 to give the dihydrocuprei(di)ne analogues. (scheme 7)
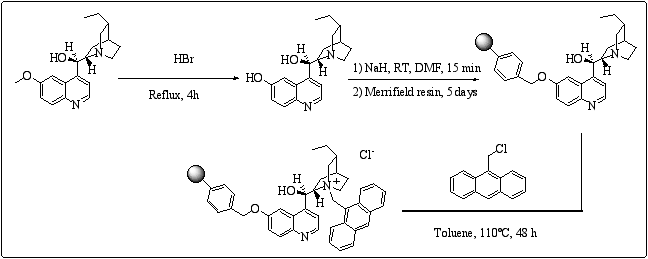
Scheme 7
: Preparation of polymer-supported type IV cinchona alkaloids.However, in spite of originality of the binding site, we did not observe any significant asymmetric induction, even if conversions and yields were good. The electronic effect and / or the steric hindrance might be responsible for the low e.e.s obtained.
4 - Type V catalysts
Anchorage of cinchona alkaloids to polymers is most commonly done through the vinyl group, those supported-derivatives being widely studied in asymmetric dihydroxylation of olefins.15 However, no report has been published concerning application in asymmetric phase-transfer catalysis. We decided to study an original method of anchorage. We applied a procedure described by Hoffmann concerning the synthesis of 10,11-didehydro cinchona alkaloids, 16 then the next step was the deprotonation by means of n-butyl lithium of the alkyne followed by the grafting on Merrifield resin. (scheme 8)
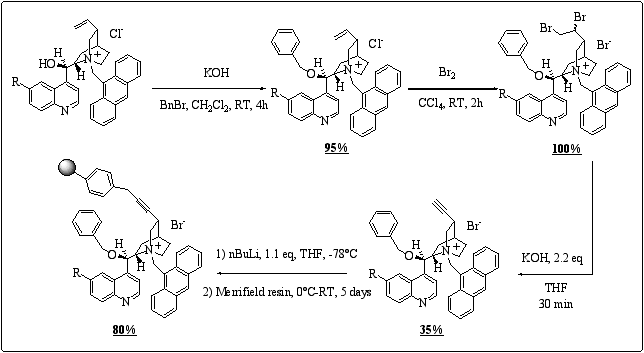
Scheme 8
: Preparation of polymer-supported type V cinchona alkaloids.The cinchonidine based polymer-supported gave an ee of 73% on benzylation of glycine Schiff base, which is a promising result, considering originality of this new kind of supported-catalyst.
In conclusion, we have shown different aspects of polymer-supported cinchona alkaloids, using different methods of preparation and sites of anchorage. Moreover in some cases, we have compared soluble and insoluble polymers. Further developments of these and related polymer-supported catalysts are in progress.
References:
1- (a) R. M. Williams, Advances in Asymmetric Synthesis, 1995, 1, 45-94. (b) R.O. Duthaler, Tetrahedron, 1994, 50, 1539-1650.
2- M.J. O’Donnell, W.D. Bennett, S. Wu. J. Am. Chem. Soc. 1989, 111, 2353-2355.
3- M.J. O’Donnell, S. Wu, C. Hoffman, Tetrahedron, 1994, 50, 4507-4518.
9- (a) B. Thierry, J-C. Plaquevent, D. Cahard, Tetrahedron: Asymmetry, 2001, 12, 983-986; (b) D. Cahard, B. Thierry, J-C. Plaquevent, ECSOC 4, Septembre 2000, Poster 32 http://reprints.net/ecsoc-4/a0032/a0032.htm.
10- B. Thierry, T. Perrard, C. Audouard, J-C. Plaquevent, D. Cahard, Synthesis, 2001, 11, 1742-1746.
11- A. M. Hafez, A. E. Taggi, T. Dudding, T. Lectka, J. Am. Chem. Soc., 2001, 123, 10853-10859.
12- C. E. Song, E. J. Roh, S. Lee, I. O. Kim, Tetrahedron: Asymmetry, 1995, 11, 2687-2694.
13- (a) H. Diaz-Arauzo, J.M. Cook, Journal of natural products, 1990, 53, 112-124. (b) E. J. Corey, J. Zhang, Organic Letters, 2001, 20, 3211-3214.
14- M. Heidelberger, W. A. Jacobs, J. Am. Chem. Soc., 1919, 41, 817.
15- (a) P. Salvadori, D. Pini, A. Petri, Synlett, 1999, 1181; (b) C. Bolm, A. Gerlach, Eur. J. Org. Chem, 1998, 21.
16- W. M. Braje, J. Frackenpohl, O. Schrake, R. Wartchow, W. Beil, H. M. Hoffmann, Helvetica Chimica Acta, 2000, 83, 777-792.