[C007]
Synthesis and photodynamic activity of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins
Inés Scalise and Edgardo N. Durantini
Departamento de Química, Universidad Nacional de Río Cuarto, Agencia Nº 3, 5800 Río Cuarto, Argentina. CONICET. E-mail: [email protected]
Abstract. 5-(4-Carboxyphenyl)-10,15,20-tris(4-methylphenyl)
porphyrin (H2P) and its metal complex with Zn(II), Pd(II), Cu(II)
and Ni(II) has been conveniently synthesized. The formation of complex produces
changes mainly in the free-base porphyrin characteristic absorption Q-bands
and in the fluorescence quantum yields (fF). The photodynamic activity of these porphyrins was analyzed using
9,10-dimethylanthracene and b-carotene as
substrates. Under these conditions, the photooxidative effect increase in the
order: NiP~CuP<<H2P<ZnP<PdP.
Introduction
One of the more recent and promising applications of porphyrins in medicine is in the detection and cure of tumors [1]. Photodynamic therapy (PDT) is an early new technique for treating cancer. After administration of a photosensitizer, which is selectively retained by tumor cells, the subsequent irradiation with visible light in the presence of oxygen specifically inactivates neoplastic cells. Basically two types of reactions can occur after photoactivation of the photosensitizer. One involves the generation of free radicals (type I photochemical reaction) and in the other, the production of singlet molecular oxygen, O2(1Dg), (type II) is the main species responsible for cell inactivation. Evidences favors the role of the type II process in cells, although the photodynamic process of the sensitizers on neoplastic tissues is still not well understood.
Adequate photosensitizers are deemed to have specific chemical and biological properties [1]. Two of the photochemical requisites are a high absorption coefficient in the visible region of the spectrum and a long lifetime of triplet excited state to produce efficiently O2(1Dg). On the other hand, the combination of hydrophobic and hydrophilic substituents in the sensitizer structure results in an intramolecular polarity axis. This property could produce a better accumulation in subcellular compartments, which is a prerequisite for an effective photosensitization [2].
Synthesis of metalloporphyrins
The condensation of 4-methylbenzylaldehyde with a large excess of pyrrole (1:48 aldehyde/pyrrole mol ratio), catalyzed by trifluoroacetic acid at room temperature, affords meso-(4-methylphenyl) dipyrromethane (Scheme 1). Flash chromatography on silica gel, using n-hexane/ethyl acetate/triethylamine (80/20/1) as eluent, yield 84% of the dipyrromethane [3].
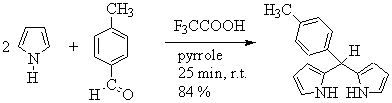
Scheme 1.
Synthesis
of meso-(4-methylphenyl) dipyrromethane
The 5-(4-carboxymethylphenyl)-10,15,20-tris(4-methylphenyl) porphyrin (ester-porphyrin) was synthesized by the acid-catalyzed condensation of a binary benzaldehyde mixture and meso-(4-methylphenyl) dipyrromethane (Scheme 2). The first reaction step was performed using catalytic among of BF3.O(Et)2 and chloroform as solvent, at room temperature. In the second step, the reaction mixture was subject to oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). The ester-porphyrin was separated by flash chromatography with high purity (17% yield) using dichloromethane/methanol gradient. Ester-porphyrin was hydrolyzed to acid-porphyrin (H2P) by heat in tetrahydrofuran/methanol/KOH medium (78% yield).
Acid-porphyrin was treated with different M+2 salts to afford the correspondent metalloporphyrins, Zn(CH3COO)2 95%, PdCl2 95%, Ni(CH3COO)2 96%, Cu(CH3COO)2 94% (Scheme 2) [4].
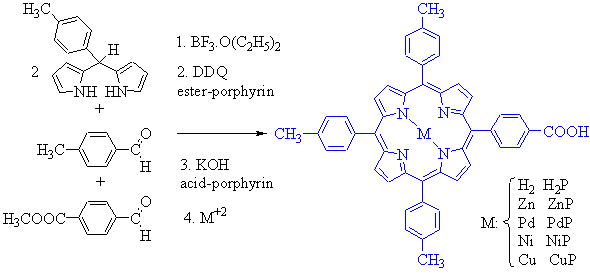
Scheme 2. Synthesis of porphyrins.
Spectroscopic studies and
porphyrin properties
Absorption spectroscopy. The absorption spectra of porphyrins in THF show the typical Soret and Q-bands, characteristic of a free-base H2P and the corresponding matalloporphyrins (Table 1, Figure 1) [4]. A sharp absorption band was obtained in every cases indicating that there is not aggregation of the porphyrin in the environment in which the photosensitizer is localized.
Table 1. The Soret band absorbance peak of porphyrins in different media.
Medium
|
H2P |
ZnP
|
PdP
|
CuP |
NiP
|
|
THF |
417.5 |
424.2 |
416.1 |
415.9 |
415.8 |
|
DMF |
419.3 |
427.0 |
418.2 |
417.3 |
417.2 |
|
heptane |
417.3 |
423.1 |
416.1 |
414.4 |
414.4 |
|
octanol |
418.9 |
426.8 |
417.6 |
415.9 |
415.9 |
|
water |
412.5 |
422.3 |
415.9 |
410.3 |
413.4 |
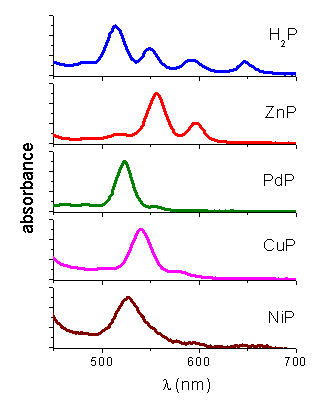
Figure 1. Absorption spectra of metalloporphyrins in THF.
Fluorescence spectroscopy. The steady-state
fluorescence emission spectra of these porphyrins were analyzed in different
media. No detectable fluorescence bands were observed for CuP and NiP. The
emission spectra of H2P, ZnP and PdP are shown in Figure 1A. The
fluorescence quantum yields (fF) of the porphyrins were calculated by steady state comparative method
using tetraphenylporphyrin (TPP) as a reference [4] (Table 2). Taking in
account the porphyrin energy of the 0-0 electronic transitions the energy levels of the singlet excited stated (1P*)
were calculated (Table 2). These
values indicate that the energy transfer from the locally excited porphyrin
singlet state (1P*) to molecular oxygen is an exothermic
process. The corrected fluorescence excitation spectra of porphyrins were recorded
in THF (Figure 2B). The excitation spectra coincide with the absorption
spectrum indicating that these porphyrins are not aggregated in this medium.
Partition coefficient. The n-octanol/water
partition coefficients of porphyrins (P) were evaluated at 25 °C (Table 2, P=[porphyrin]o/[porphyrin]w).
The lipophilic character increases in these metalloporphyrin with respect to
free-base H2P, being PdP the less soluble porphyrin in water.

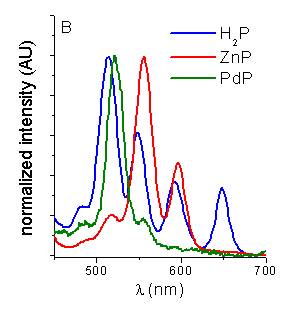
Figure 2. A. Emission spectra of porphyrins in THF (lexc=550nm). B. Excitation spectra of porphyrins in THF (lem=720, 660, 610 nm respectively for H2P, ZnP and PdP).
Table 2. Fluorescence quantum yields (fF) and partition coefficients of porphyrins.
Porphyrin
|
fF |
Es (eV) |
P
|
Log P
|
|
H2P |
0.11 ±0.01 |
1.90 |
4.48 |
0.65 |
|
ZnP |
0.054 ±0.002 |
2.07 |
50.2 |
1.70 |
|
PdP |
(1.3±0.2)x10-4 |
2.12 |
85.6 |
1.93 |
|
CuP |
- |
|
31.3 |
1.50 |
|
NiP |
- |
|
49.1 |
1.69 |
Singlet
molecular oxygen, O2(1Dg), production
Photooxidation of 9,10-dimethylanthracene (DMA). The aerobic irradiations, with monochromatic light, of photosensitizers in THF were performed in the presence of 9,10-dimethylanthracene (DMA). This substrate quenches O2(1Dg) by exclusively chemical reaction [5]. A time-dependent decrease in the DMA concentration was observed by following a decrease in its absorbance (Figure 1). From these plots the values of the observed rate constant (kobs) were calculate for DMA (Table 3). The quantum yield of O2(1Dg) production (FD) was calculated from the slopes of the plots for the porphyrins compared with the corresponding slope obtained for the reference (TPP). A considerable increase in FD was obtained by forming a metal complex of porphyrin with Zn and Pd, while no DMA reaction was detected using ether CuP or NiP as sensitizer.
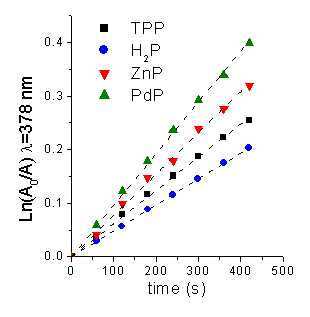
Figure 3. First-order plots for the photooxidation of DMA (45 mM) photosensitized by different porphyrins in THF.
Table 3. Observed rate constant (kobsDMA) for DMA and O2(1Dg) quantum yield (FD) of porphyrins in THF.
Porphyrin
|
kobsDMA (s-1) |
FD |
|
TPP |
(6.28±0.1) x10-4 |
0.62 |
|
H2P |
(4.8±0.1) x10-4 |
0.48 |
|
ZnP |
(7.7±0.1) x10-4 |
0.77 |
|
PdP |
(9.6±0.1) x10-4 |
0.96 |
Photoprotector effect of b-carotene (Car). Evidence for O2(1Dg) pathway was provided performing the photooxidation reaction of DMA in presence of Car [1]. Carotenoids are efficient quenchers of O2(1Dg) via both energy transfer and chemical process [6]. Under these conditions both substrates are photooxidated (Figura 4). A time-dependent decrease in the substrate concentration was observed by following a decrease in its absorbance (Figure 5 and 6, for DMA and Car, respectively). In all cases, the reaction rate (kobsDMA) of DMA is diminished with a quenching efficience (hq) of ~0.90 (Table 4). Taking into account the Stern-Volmer equation in the presence of Car (t0/t =1+t0kq[Car], t0=2.32x10-5s-1, kq= in THF, [Car]=14 mM) a value of t/t0 = 0.10 was calculated. This result show that under these conditions a 90% of quenching should be expected when O2(1Dg) mediation is the responsible of photooxidation process.
To evaluate the reaction rate constant of Car photooxidation (krCar) in this system, DMA was used as actinometer in the same experimental conditions. From the ratio of the first-order slopes between the Car and the actinometer (krCar = kobsCar krDMA / kobsDMA, krDMA=5x107 s-1M-1 [7]), the values of krCar were calculated in THF using H2P, ZnP and PdP as sensitizers (Table 4).
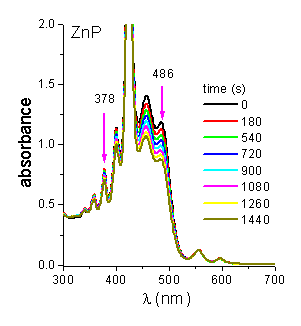
Figure 4. Spectra for the photooxidation of DMA (45 mM) and Car (14 mM) sensitized by ZnP at different irradiation times in THF.
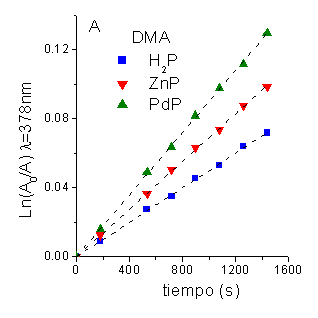
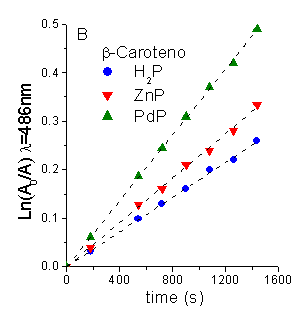
Figure 5. First-order plots for the photooxidation of: A. DMA (45 mM) and B. Car (14 mM) photosensitized by different porphyrins in THF.
Table 4. Kinetic parameters for the photooxidation of DMA and Car sensitized by porphyrins in THF.
|
Porphyrin |
kobsDMA+Car (s-1) |
kobsCar (s-1) |
krCar (s-1M-1) |
hq |
|
H2P |
(5.0±0.1) x10-5 |
(1.8±0.1) x10-4 |
(1.8±0.2) x107 |
0.90 |
|
ZnP |
(6.9±0.1) x10-5 |
(2.3±0.1) x10-4 |
(1.5±0.2) x107 |
0.91 |
|
PdP |
(9.0±0,1) x10-5 |
(3.4±0.1) x10-4 |
(1.7±0.2) x107 |
0.91 |
Conclusions
The following steps were used sequentially in the synthesis: 1) meso-(4-methylphenyl) dipyrromethane was formed from 4-methylbenzaldehyde and excess pyrrole catalyzed by acid, 2) The ester-porphyrins bearing one 4-carboxymethylphenyl group and three identical peripheral methyl groups, were obtained with appreciable yields of 17% by condensation of dipyrromethane with an appropriate binary mixture of benzaldehydes. 3) The resultant ester-porphyrins was hydrolyzed to acid-porphyrin and 4) the treatment of free-base porphyrin with metal(II) salt produces the corresponding porphyrin metal complex.
The formation of complex produce changes in the Q-bands and a diminishing in the emission of fluorescence. No emission was detected for CuP and NiP. This effect is can be disadvantage for detection and quantification. The photodynamic activity of these porphyrins in the presence of DMA increase in the order: NiP~CuP<<H2P<ZnP<PdP. Similar tendency was observed when the photooxidative reaction was performed in the presence of Car as O2(1Dg) quencher. Also, the quenching effect of Car is in concordance with a tipe II photoreaction process. Although many other factor can contribute in cellular systems, an increase in the photodamage could be expected for porphyrins with high O2(1Dg) generation, such as ZnP and PdP.
Acknowledgements. Authors thank Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina, Agencia Nacional de Promoción Científica y Técnica, TWAS and Fundación Antorchas for financial support. E.N.D. is Scientific Members of CONICET.
References
1. R. Bonnett, in Chemical Aspects of Photodynamic Therapy, Advanced Chemistry Texts, Gordon and Breach Science Publishers, Amsterdam, 2000.
2. A. Weitemeyer, H. Kliesch, U. Michelsen, A. Hirth and D. Wöhrle, in Photodynamic Tumor Therapy, 2nd and 3rd Genaration Photosensitizers, Chapter 2.6: Unsymmetrically Substituted Porphyrazines, ed. J. G. Moser, Harwood Academic Publishers, Amsterdam, 1998; p. 87-99.
3. F. Fungo, L. A. Otero, L. Sereno, J. J. Silber, E. N. Durantini, J. Mater. Chem. 2000, 10, 645.
4. E. Milanesio, M. G. Alvarez, E. I. Yslas, C. D. Borsarelli, J. J. Silber, V. Rivarola, E. N. Durantini, Photochem. Photobiol. 2001, 74, 14.
5. C. D. Borsarelli, E. N. Durantini, N. A. García, J. Chem. Soc. Perkin Trans 2 1996, 2009.
6. M. A.
Montenegro, M. A. Nazareno, E. N. Durantini, C. D. Borsarelli, Photochem.
Photobiol. 2002,
75, 353.
7. M. La Penna, M. G. Alvarez, E. I. Yslas,
V. Rivarola, E. N. Durantini, Dyes and Pigments 2001, 49, 75.