[E005]
 |
Microwave enhanced synthesis of bowl-shaped triimides with C3-symmetry |
|
[E005]
 |
Microwave enhanced synthesis of bowl-shaped triimides with C3-symmetry |
|
The use of microwave irradiation for the enhancement of imide formation by pyrolisis of amine-dicarboxylic acid salts have been atracting our interest in the las few years.1
Here we report our preliminar findings in the study of triimides of all-cis-1,2,3,4,5,6-cyclohexanecarboxylic acid (1). Few reports have been done on the synthesis of triimides of 1, those reports follow either trimerization of maleimides2 or the heating at high temperatures of salts.3 Due to the interest of this kind of structures we decided to take a new insight on their preparation.
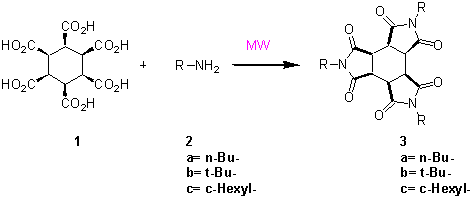
Scheme 1
In our method, to synthetize triimides an aqueous solution of 1 equivalent of hexacarboxylic acid 1 and 3 equivalents of the corresponding amine (2a-c) was irradiated in an open vessel with microwaves during 15 minutes. In all cases the corresponding imide was obtained in very good yield (table 1) of pure imides 3a-c.
Table 1
|
entry |
amine |
triimide |
yield |
|
1 |
2a |
3a |
quantitative |
|
2 |
2b |
3b |
quantitative |
|
3 |
2c |
3c |
95% |
Minimized structures of triimides 3b4
| Fig.1 Chair
conformation
Heat of formation= -206.09076 kcal/mole, Mopac2000-MINDO/3. |
| Fig.2 Boat
conformation
Heat of Formation: -203.40584 kcal/mole, Mopac2000-MINDO/3 |
| Fig. 3
cis-1,2,3,4-trans-5,6-triimde
a possible product from trimerization of maleimide Heat of Formation: -207.67718 kcal/mole, Mopac2000-MINDO/3 |
We think our method is a direct and easy to get bowl-shaped imides with C3-symmetry and it is superior to the trimerization of maleimides which can render mixtures of diastereomers.
Connolly Molecular surfaces showing the bowl shape
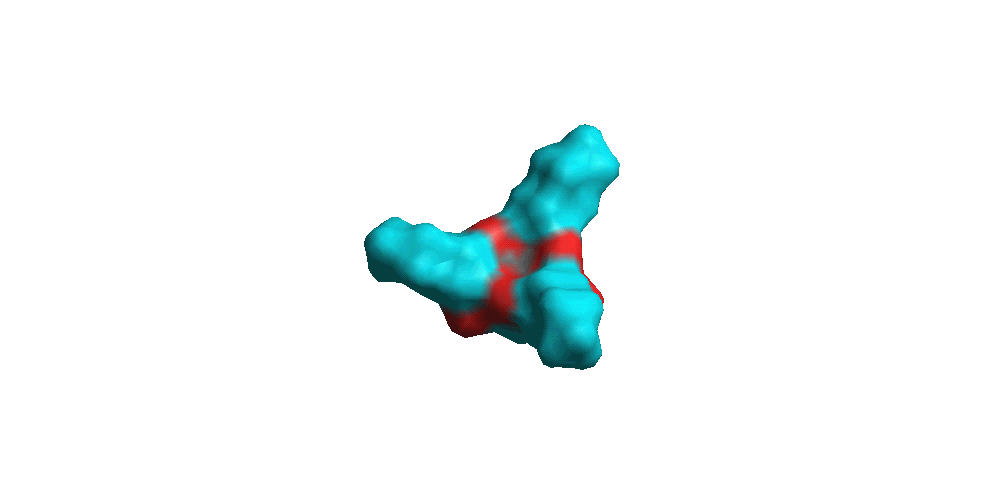 |
3a
|
 |
3b
|
 |
3c
|
Acknowledgements:
Xunta de Galicia ( infraestructura PR405A 98/59-0 and PGIDT01PXI26203PR) for financial support.
1.- Seijas, J. A.; Vázquez-Tato, M. P.; Matínez, M. M.; Nuñez-Corredoira, G. J. Chem. Res. (S), 1999, 420-421.Seijas, J. A.; Vázquez-Tato, M. P.; González-Bande, C.; Martinez, M. M.; Pacios-López, B Synthesis, 2001, 999-1000.
2.-Wagner-Jauregg, Th.; Ahmed, Q.; Pretsch, E. Helv. Chim. Acta 1973, 56, 1406-13. . Lulukyan, K. K.; Poshotyan, A. Zh.; Agbalyan, S. G. Arm. Khim. Zh. 1981, 34, 237-41. Chem. Abstr. 1981, 95, 132627.
3.-Nottes, G.; Nohe, H..German Pat. DE 241778, 1975; Chem. Abstr. 1976, 84, 92567.
4.- Calculations were performed with the implementation CSMopac in the
program Chem3d Ultra 6.0, CambridgeSoft CoroporationTM. In order to wiev this 3D
structures you will need to have instaled Chime plug-in in your browser ![]()