7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[C002]
Synthesis and photodynamic activity of tetracationic and non-charged zinc phthalocyanine derivatives in homogeneous and biological media
Inés Scalise and Edgardo N. Durantini
Departamento de Química, Universidad Nacional de Río Cuarto, Agencia Nº 3, 5800 Río Cuarto, Argentina. CONICET. E-mail: [email protected]
Abstract. A new tetracationic zinc phthalocyanine derivative (ZnPc 2) has been conveniently synthesized fron a non-charged phthalocyanine (ZnPc 1). The spectroscopic properties and the photodynamic activity of these phthalocyanines (ZnPcs) were compared en homogeneous media. Both ZnPcs show the characteristic absorption Q-bands (~680 nm) and a fluorescence quantum yields (fF) of ~0.16. The photodynamic activity of these ZnPcs was analyzed using 9,10-dimethylanthracene as substrates. Under these conditions, the photooxidative effect was similar to that of zinc phthalocyanine. However, only ZnPc 2 produce efficient photoinactivation of Escherichia coli. These results show that the tetracationic ZnPc 2 can be a promising phototherapeutic agent with potential applications in Gram-negative bacteria and tumor cell inactivation by photodynamic therapy.
There is an urgent need for fresh approaches to the treatment of bacterial infections because of changing patterns of infectious disease and the emergence of bacterial strains resistant to current antibiotics [1]. Photosensitive tetrapyrrolic molecules, such as phthalocyanines, are known to accumulate and be retained by a variety of rapidly proliferating tissues and cells, including microorganisms. Subsequent photoactivation of these molecules with visible light in the presence of oxygen leads to cell inactivation as a result of singlet oxygen-mediated damage. This methodology, photodynamic therapy (PDT), has proven valuable in the treatment of a variety of hyperproliferative conditions, including small solid tumors and macular degeneration of the retina [2]. Pathogenic microorganisms growing in vivo as localized foci of infection, on skin or on accessible mucous membranes, could be good candidates for photodynamic destruction.
Basically two types of reactions can occur after photoactivation of the photosensitizer. One involves the generation of free radicals (type I photochemical reaction) and in the other, the production of singlet molecular oxygen, O2(1Dg), (type II) is the main species responsible for cell inactivation. Both reactions can occur simultaneously and the ratio between two processes depends of the sensitizer, substrate and the nature of the medium [2,3].
Gram-positive bacteria are susceptible to photosensitized porphyrin-induced antibacterial activity [4]. Gram-negative bacteria exhibit induced damage with light only when an agent, which stimulates the translocation of sensitizers through the membranes, is added to the to the cultures. However, cationic sensitizers have shown to photoinduce direct inactivation of Gram-negative bacteria without the presence af an additional permeabilization agent [5,6].
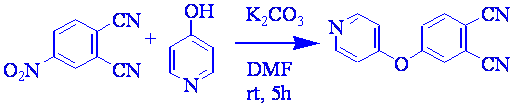
The synthesis requires three steps: 1) phthalonitrile derivative was obtains by the substitution reaction of 4-nitrophthalinitrile with 4-hydroxypyridine in basic medium with a yield of 73% (Scheme 1); 2) the condensation of phthalonitrile derivative in presence of DBU and Zn(II) acetate produces the correspondent ZnPc 1 yielding 75 % (Scheme 2); 3) treatment with methyl iodide of ZnPc 1 affords tertacationic ZnPc 2 with a yield of 96 % (Scheme 2).

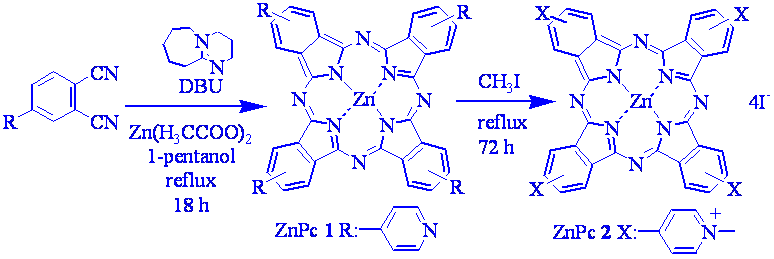
Scheme 2. Synthesis of ZnPn 1 and 2.
Absorption spectroscopy. The absorption spectra of ZnPcs in N,N-dimethylformamide (DMF) and methanol show the typical Soret and Q-bands (Figure 1) [2]. The position of the maxima and the relative intensities of the bands are typical of monomeric phthalocyanines. However, when ZnPcs are dissolved in PBS, the Q bands undergo a significant broadening. These spectral features are characteristic of aggregated phthalocyanines [6].
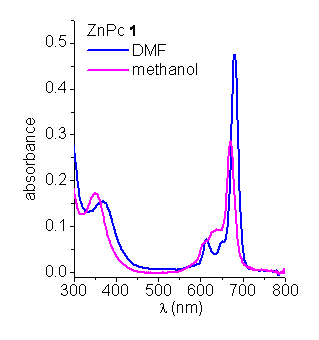
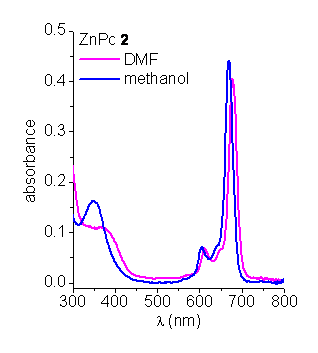
Figure 1. Absorption spectra of ZnPcs in DMF and methanol.
Fluorescence spectroscopy. The steady-state fluorescence emission spectra of these ZnPcs were analyzed in DMF and methanol (Figure 2). They shows a typical shape for phthalocyanines with a peak at ~ 688 nm in DMF. The fluorescence quantum yields (fF) of the ZnPcs were calculated by steady state comparative method using zinc phthalocyanine (ZnPc) as a reference (fF ZnPc=0.28 in DMF, Table 1).
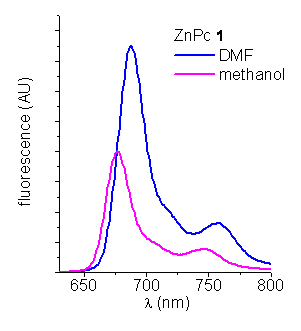
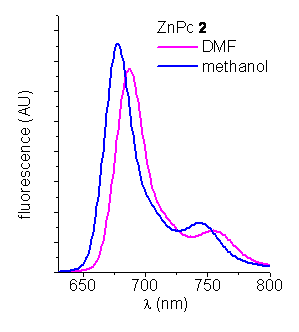
Figure 2. Emission spectra of ZnPcs in DMF and methanol (lexc=612 nm).
Table 1. Fluorescence quantum yields (fF) in DMF and partition coefficients of ZnPcs.
Phthalocyanine |
fF |
P |
Log P |
|
ZnPc 1 |
0.16±0.01 |
1.60 |
0.204 |
|
ZnPc 2 |
0.16±0.01 |
0.88 |
-0.055 |
The corrected fluorescence excitation spectra of porphyrins were recorded in THF (Figure 3). The excitation spectra coincide with the absorption spectrum indicating that these porphyrins are mainly not aggregated in this medium.

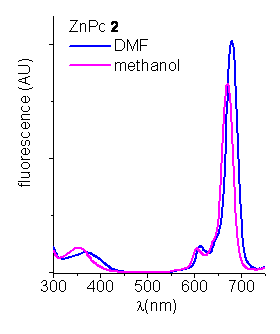
Figure 3. Excitation spectra of ZnPcs in DMF and methanol (lem=760).
Partition coefficient. The n-octanol/water partition coefficients of ZnPcs were evaluated at 25 °C (Table 1, P=[porphyrin]o/[porphyrin]w). As expected, the hydrophilic character increases in ZnPc 2 according with the presence of ionic substituents.
Production of singlet molecular oxygen, O2(1Dg)
Photooxidation of 9,10-dimethylanthracene (DMA). The aerobic irradiations, with monochromatic light, of photosensitizers in DMF were performed in the presence of 9,10-dimethylanthracene (DMA). This substrate quenches O2(1Dg) by exclusively chemical reaction [3]. A time-dependent decrease in the DMA concentration was observed by following a decrease in its absorbance (Figure 4). From these plots the values of the observed rate constant (kobs) were calculate for DMA (Table 2). The quantum yield of O2(1Dg) production (FD) was calculated from the slopes of the plots for the ZnPcs compared with the corresponding slope obtained for the reference ZnPc (Table 3). The values of FD are very similar for these phthalocyanines in DMF.
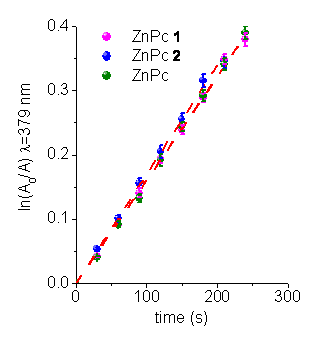
Figure 4. First-order plots for the photooxidation of DMA (45 mM) photosensitized by different ZnPcs in DMF.
Table 3. Observed rate constant (kobsDMA) for DMA and O2(1Dg) quantum yield (FD) of porphyrins in THF.
Porphyrin |
kobsDMA (s-1) |
FD |
|
ZnPc |
(1.61±0.05) x 10-3 |
0.50 |
|
ZnPc 1 |
(1.61±0.05) x 10-3 |
0.50 |
|
ZnPc 2 |
(1.70±0.05) x 10-3 |
0.53 |
Control studies showed that the incubation of E. coli cells with 10 mM for 30 min in the dark has no effect on cell survival. On the other hand, a large difference in photosensitivity is observed when E. coli cells are irradiated with visible light after washing step (Figure 5). The result shows that only about 0.01 % of E. coli cells survival when the Gran-negative bacteria are treated with tetracationic ZnPc 2.
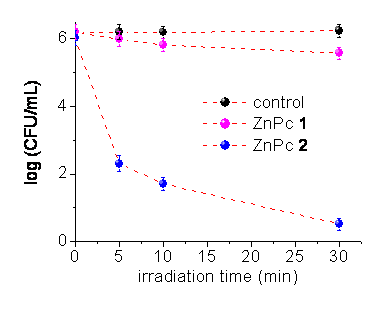
Figure 5. Photoinactivation of Echerichia coli treated with sensitizer (10 mM, 30 min in the dark), washed and irradiated with visible light (~80 mW/cm2), .
� Tetracationic ZnPc 2 was conveniently synthesized by methylation of ZnPc 1, which was obtained from the condensation of phthalonitrile derivative in presence of DBU and Zn(II) acetate.
� The spectroscopic properties, absorption and fluorescence, of ZnPc 2 are appropriated for its use as photosensitizer.
� The O2(1Dg) production of both ZnPcs are similar to that of ZnPc en DMF.
� A higher phototoxic effect on E. coli was found for ZnPc 2 with respect to non-charged ZnPc 1.
These results show that the cationic ZnPc 2 offers a promising molecular architecture for photosensitizer agents with potential applications in Gram-negative bacteria inactivation by photodynamic treatment. Further in vitro studies concerning the mechanism of cell photoinactivation produced by ZnPc 2 are presently in progress in our laboratory.
Acknowledgements. Authors thank Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina and Fundación Antorchas for financial support. E.N.D. is Scientific Members of CONICET.
1. P. W. Taylor, P. D. Stapleton and J. P. Luzio Drug Discovery Today 2002, 7, 1086.
2. R. Bonnett, in Chemical Aspects of Photodynamic Therapy, Advanced Chemistry Texts, Gordon and Breach Science Publishers, Amsterdam,
2000.
3. E. Milanesio, M. G. Alvarez, E. I. Yslas, C. D. Borsarelli, J. J. Silber, V. Rivarola and E. N. Durantini Photochem. Photobiol. 2001, 74, 14.
4. Y. Nitzan and H. Ashkenazi Curr. Microbiol. 2001, 42, 408.
5. A. Minnock, D. I. Vernon, J. Schofield, J. Griffiths, J. H. Parish, S. B. Brown J. Photochem. Photobiol. B: Biol. 1996, 32, 159.
6. A. Segalla, C. D. Borsarelli, S. E. Braslavsky, J. D. Spikes, G. Roncucci, D. Dei, G. Chiti, G. Jori, E. Reddi Photochem. Photobiol. Sci. 2002, 1,
641.