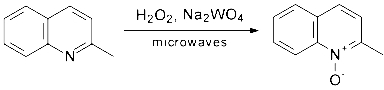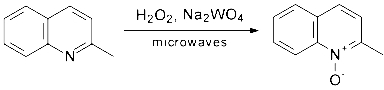7th International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30
November 2003
[E006]
Microwave assisted oxidation of
azaheterocyclics
Marcin Lukasiewicz, Dariusz Bogdal
Department
of Polymer Science, Politechnika Krakowska,
ul. Warszawska 24 31-155 Krakow,
Poland
e-mail: [email protected]
ABSTRACT
The microwave assisted oxidation of nitrogen containing
heterocyclic compounds was presented. The hydrogen peroxide was used as an
oxidant employing sodium tungstate as a catalyst and the reaction was carried
out in the multimode microwave reactor, at the boiling of the reaction mixture.
As a result the appropriate N-oxide with a very good yield and purity were
obtained.
KEYWORDS
microwave, oxidation, hydrogen peroxide, N-oxide, azaheterocylic
compound
INTRODUCTION
The recent development in so called "green chemistry" shows
that alternative methods of carrying out chemical transformation could minimize
the environmental harmfulness of classical reactions. One of the most popular
and interesting approach on this field is employing the microwave energy for
conducting many chemical transformations
[1].
Using this method the reaction mixture is irradiated by microwaves with the
frequency of 2,45GHz (usually). The interaction of the matter with such kind of
electromagnetic waves results in: higher speed of heating (because of volumetric
heating)
[2],
different distribution of temperature
[3]
and in the case of the mixture containing compounds which differ with polarity
in selective heating of more polar molecules
[4].
Examples of microwave assisted synthesis already described in the literature
show much shorter reaction time and very often the higher selectivity of desired
products
[1].
From the other hand the application of hydrogen peroxide as an oxidant could
also improved the environmental aspect of organic syntheses becouse of its high
oxidation potential and non-toxic reduction product (i.e. water) [5].
The combination of accelerating of chemical reactions and using such
"environment friendly" reagents (oxidants) seems to enlarge the field of "green
organic chemistry".
As a continuation of our earlier research on microwave assisted oxidation
reactions [6]
we now report the microwave oxidation of some azaheterocyclic compounds using
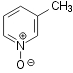
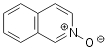
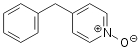
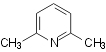
hydrogen peroxide as the oxidant (shown on Scheme 1 for oxidation of quinaldine)
which is a very important transformation on the field of obtaining substituted
azaheterocyclic compounds.
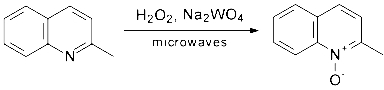 Scheme 1.
Oxidation of quinaldine
Scheme 1.
Oxidation of quinaldine
EXPERIMENTAL
All the chemicals were purchased from Aldrich and used as
received. The reactions were carried out in a multimode microwave reactor with a
continuous power regulation (PLAZMATRONIKA, Poland), which is equipped with
magnetic stirrer and two inlets on the top and one side of the reactor. The
inlets allowed applying an upright condenser and introducing a fibre-optical
sensor (ReFlex, Nortech) which was used to control temperature during microwave
experiments. IR spectra were recorded on FT-IR BIORAD FTS-165 spectrophotometer
as liquids on NaCl disks. H-NMR spectra were collected on Tesla 487C (80MHz)
spectrometer using TMS as an internal standard. GC/MS spectra were determined on
GC/MS 5890 SERIES II HEWLETT-PACKARD gas chromatograph equipped with Ultra 2
(25m x 0.25mm x 0.25 mm) column with HEWLETT-PACKARD 5971 Series Mass Selective
Detector.
General method for the oxidation
All the reactions
were carried out according the oxidation procedure given for quinaldine, which
was representative of the general procedure employed for microwave conditions.
Quinaldine (20mmol, 2,86g) was placed in a 50 mL round-bottom reaction flask.
Then hydrogen peroxide (30mmol, 1,02g) as a 30% water solution was added
following the sodium tungstate (0,3mmol 0,1g) which was used as a catalyst. Next
the magnetically stirred suspension was irradiated (250W of microwave power) in
an open vessel at reflux using multimode microwave reactor (Plazmatronika,
Poland) during 30min. After completion of the process products was isolated by
means of high performance liquid chromatography what afford 2,38g (75%) of
quinaldine N-oxide. In order to proove the structure of the product the
following characterisation were done: FTIR, HNMR and MS.
RESULTS AND DISCUSION
LITERATURE
- For relevant papers and reviews on microwave assisted
chemical reactions see: R. A. Abramovitch; Org. Prep. Proc. Int.;
1991, 23, 683; S. Caddick; Tetrahedron; 1995, 51, 10403;
C. R. Strauss, R. W. Trainor; Aust. J. Chem.; 1995, 48, 1665; A.
Loupy, A. Petit, J. Hamelin, F. Texier-Boullet, P. Jacquault, D. Mathe;
Synthesis; 1998, 1213; S. Deshayes, M. Liagre, A. Loupy, J. L.
Luche, A. Petit; Tetrahedron; 1999, 55, 10851; R. S. Varma;
Green Chem.; 1999, 43; P. Lidstrom, J. Tierney, B. Wathey, J.
Westamn; Tetrahedron; 2001, 57, 9225; L. Perreux, A. Loupy;
Tetrahedron; 2001, 57, 9199; A. K. Bose, M. S. Manhas, S. N.
Ganguly, A. H. Sharma, B. K. Banik; Synthesis; 2002, 1578-1591;
A. Loupy (Ed.); Microwaves in Organic Sytnhesis, , Wiley-VCH, Weinheim,
2002.
- C. Gabriel, S. Gabriel, D. Mingos; Chem. Soc. Rev.;
1997, 27, 213
- R. Saillard, M. Poux, J. Berlan; Tetrahedron;
1995, 14, 4033
- H. Kingstaon, S. Haswell; Microwave-Enhanced
Chemistry; ACS, 1997
- M. Lukasiewicz, J. Pielichowski; Przem. Chem.;
2002, 8, 509
- D. Bogdal, M. Lukasiewicz; Synlett; 2000, 1,
143; D. Bogdal,M. Lukasiewicz, J. Pielichowski, A. Miciak and Sz. Bednarz;
Tetrahedron; 2003, 59, 649; D. Bogdal , M. Lukasiewicz;
Intern. Conf. on Microwave Chem.; Antibes; France 2000; D. Bogdal , M. Lukasiewicz; 5th Electronic Conference in
Synthetic Organic Chemistry, ECSOC, 2001; D. Bogdal , M. Lukasiewicz; 6th Electronic Conference in
Synthetic Organic Chemistry, ECSOC, 2002; D. Bogdal , J.
Pielichowski, M. Lukasiewicz; 1st International Conference "Microwave in
Chemistry"; Gainsville; USA, 2003