Microwave-Assisted Hydroxylation of
Simple Olefins
Marcin Lukasiewicz,
Dariusz Bogdal
Department of Polymer Science, Politechnika Krakowska,
ul.
Warszawska 24 31-155 Krakow, Poland
e-mail: [email protected]
ABSTRACT
The hydroxylation of some simple cyclic alkenes i.e.
cyclohexene and cyclooctene by hydrogen peroxide using microwaves as an energy
source is described. The reaction results in the introduction of both hydroxy
and alcoxy group depending on the alcohol used as a solvent.
KEYWORDS
microwaves, hydrogen peroxide, hydroxylation, alkenes.
INTRODUCTION
The introduction of hydroxyl group into the organic molecules
results in the obtaining of the very interesting group of compounds, which finds
many application as both final products and intermediates
[1]
and was carried out in many different maners including apllication of hydrogen
peroxide as the oxidant and tungstic acid as the catalyst
[2].
The one of most interesting compounds on these field is cyclohexene, which could
be transformed into
w-caprolactame (via a cyclohexanol
stage) or directly into adypic acid
[3].
Unsaturated compounds could also be oxided into the corresponding oxiranes and
the selectivity of the reaction depends on the reaction conditions
[4].
From the other hand the application of microwaves in synthetic organic
chemistry shown recently in many publication [5]
leads to the conclusion, that this kind of the energy transport could have a big
influence on both rate of the reaction and its selectivity. Such phenomenon is a
consequence of the interaction of microwaves with the matter by dielectric and
conducting mechanism [6].
As a continuation of our earlier research on microwave assisted oxidation
reactions [7]
we now report the microwave hydroxylation of some simple olefins using hydrogen
peroxide as the oxidant and solid tungstic acid as a catalyst.
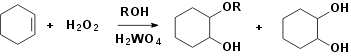
EXPERIMENTAL
All the chemicals were purchased from Aldrich and used as
received and the reactions were carried out in two phase system containing solid
tungstic acid and the solution of hydrogen peroxide and substrate in different
alcohols (Scheme 1). The processes were carried out in a multimode and monomode
microwave reactor with a continuous power regulation (PLAZMATRONIKA, Poland),
which is equipped with magnetic stirrer and two inlets on the top and one side
of the reactor. The inlets allowed applying an upright condenser and introducing
a fibre-optical sensor (ReFlex, Nortech) which was used to control temperature
during microwave experiments. IR spectra were recorded on FT-IR BIORAD FTS-165
spectrophotometer as liquids on NaCl disks. H-NMR spectra were collected on
Tesla 487C (80MHz) spectrometer using TMS as an internal standard. GC/MS spectra
were determined on GC/MS 5890 SERIES II HEWLETT-PACKARD gas chromatograph
equipped with Ultra 2 (25m x 0.25mm x 0.25 mm) column with HEWLETT-PACKARD 5971
Series Mass Selective Detector.
The comparable experiments were performed applying
conventional conditions (thermostated water bath). All the experiments were
carried out using tempereature and power programs listed in Table 1. In the
hydroxylation of cyclohexene in methanol, which is representative for all the
reactions the cyclohexene (25mmol) were dissolved in the methanol
(20ml).
Table 1. Power and temperature programs for the
hydroxylation of olefins
| Reactor |
Program |
multimode reactor
Plazmatronika
Poland |
power: 0-110W
temperature: boiling of the
mixture |
monomode reactor
Plazmatronika
Poland |
power: 0-75W
temperature: boiling of the
mixture |
| conventional heating |
temperature: boiling of the
mixture |
Then solid tungstic acid were
added (1,3mmol). The mixture was then irradiated by microwave or heated
conventionally and after its reaches 60°C, hydrogen peroxide (26mmol, 2,65ml of
30% water solution) were added. The mixture were then stirred and irradiated
(MW)/heated up (conventionally) to the boiling under the reflux. After the
reaction is finished the mixture were extracted by ether. The organic layer was
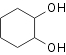
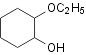
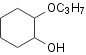
evaporated resulting the crude mixture of 1,2-dihydroxycyclonehexan and
1-hydroxy-2-methoxy-cyclohexan. Products were separated by the vacuum
destilation and characterized by ftir, HNMR and MS spectroscopy.
As it was expected
[2]
during the research we have obtained a series of cyclohexene derivatives with
yields listed in Table 2. In the case of cycloocten (reaction carried out in
methanol) we have observed that except of appropriate diole (15%) and
hydroxyalcoxy compound (7%) the main product is the 9-oxabicyclo-(6.1.0)nonane
(37%). The oxirane like compounds are known as intermediates during the
hydroxylation process, but were not detected in case of cyclohexene. In the case
of cyclooctene oxirane observed as a main product seems to be the consequence of
higher stability of the eight atoms ring.
Table 2. Hydroxylation of cyclohexene
| solvent |
microwaves |
product |
conventional |
| Yield [%] |
Time [min] |
Yield [%] |
Time [min] |
| methanol |
56 |
60 |
 |
50 |
120 |
| 25 |
60 |
 |
13 |
120 |
| ethanol |
41 |
60 |
 |
25 |
120 |
| 29 |
60 |
 |
25 |
120 |
| 1-propanol |
0 |
60 |
 |
0 |
120 |
| 27 |
60 |
 |
20 |
120 |
| benzyl alcohol |
0 |
120 |
 |
0 |
150 |
| 20 |
120 |
 |
20 |
150 |
In the case of simple linear
alkenes i.e. 1-dodecene, 1-decene, 1-octene and 9-allilo-9-H-carbazole neither
oxiranes nor hydroxy-like compounds were detected. In cyclohexene hydroxylation
no change in the product distribution were observed. The comparison of the
microwave and conventional conditions shows that the slightly higher final
yields of the products in microwave conditions, but the reaction times for such
kind of energy transfer were up to two times shorter. The influence of
microwaves distribution (i.e. monomode and multimode reactors) shows no
differences. The application of different alcohols as a solvents result in
decreasing of the yields of 1-hydroxy-2-alcoxycyclohexane following by the
slightly increase in 1,2-dihydroxycyclohexane. For 1-propanol and benzylic
alcohol no alcoxy product were detected, but also for these transformation the
shortenion of the microwave reaction times were observed.
As a conclusion we would like to emphasise, that described hydroxylation
could be carried out more efectively (the mining of the shortening of the
reaction times) in the presence of microwaves however no change in selectivity
is observed.
LITERATURE
- B.M. Trost; Comprehensive of Organic Synthesis
(Oxidation), Ed.; Pergamon; New York, 1991
- G. Payne, C. Smith; J. Org. Chem.; 1957, 22,
1682
- K. Sato, M. Aoki, J. Takagi, R. Noyori, Science;
1998, 281, 1646
- K. Joergensen; Chem. Rev.; 1989, 3, 431
- For relevant papers and reviews on microwave assisted
chemical reactions see: R. A. Abramovitch; Org. Prep. Proc. Int.;
1991, 23, 683; S. Caddick; Tetrahedron; 1995, 51, 10403;
C. R. Strauss, R. W. Trainor; Aust. J. Chem.; 1995, 48, 1665; A.
Loupy, A. Petit, J. Hamelin, F. Texier-Boullet, P. Jacquault, D. Mathe;
Synthesis; 1998, 1213; S. Deshayes, M. Liagre, A. Loupy, J. L.
Luche, A. Petit; Tetrahedron; 1999, 55, 10851; R. S. Varma;
Green Chem.; 1999, 43; P. Lidstrom, J. Tierney, B. Wathey, J.
Westamn; Tetrahedron; 2001, 57, 9225; L. Perreux, A. Loupy;
Tetrahedron; 2001, 57, 9199; A. K. Bose, M. S. Manhas, S. N.
Ganguly, A. H. Sharma, B. K. Banik; Synthesis; 2002, 1578-1591;
A. Loupy (Ed.); Microwaves in Organic Sytnhesis, , Wiley-VCH, Weinheim,
2002.
- H. Kingstaon, S. Haswell; Microwave-Enhanced
Chemistry; ACS, 1997
- D. Bogdal, M. Lukasiewicz; Synlett; 2000, 1,
143; D. Bogdal,M. Lukasiewicz, J. Pielichowski, A. Miciak and Sz. Bednarz;
Tetrahedron; 2003, 59, 649; D. Bogdal , M. Lukasiewicz;
Intern. Conf. on Microwave Chem.; Antibes; France 2000; D. Bogdal , M. Lukasiewicz; 5th Electronic Conference in
Synthetic Organic Chemistry, ECSOC, 2001; D. Bogdal , M. Lukasiewicz; 6th Electronic Conference in
Synthetic Organic Chemistry, ECSOC, 2002; D. Bogdal , J.
Pielichowski, M. Lukasiewicz; 1st International Conference "Microwave in
Chemistry"; Gainsville; USA, 2003





