[E008]
THE COMPARATIVE STUDY OF THE KINETICS OF KNOEVENAGEL CONDENSATION UNDER MICROWAVE AND CONVENTIONAL CONDITIONS. Part III.
Szczepan Bednarz, Dariusz Bogdal*
*e-mail: [email protected]
Department of Polymer Science and Technology Politechnika Krakowska ul. Warszawska 24, 31-155 Krakow, Poland
______________________________________________________________________________________________
Abstract
The
reaction of the Knoevenagel condensation of salicylaldehyde,
diethylmalonate in the presence of 4-piperydinopiperidyne as a catalyst in
toluene as a solvent has been studied. The research were focused on the
influence of microwave radiation on the reaction kinetics.
Keyworts
microwave irradiation, microwave
effect, chemical kinetics, knoevenagel reaction
_____________________________________________________________________________________________
In
the recent articles [1][2]
we presented comparative study of the Knoevenagel condensation under
microwave radiation and conventional heating where rate enhancement of
investigated reaction was observed. We studied kinetic of reaction between
salicylaldehyde and diethylmalonate in the presence of piperidyne as a
catalyst in toluene as a solvent. To settle the matter existence of
non-thermal microwave effect in Knoevenagel condensation a similar
reaction has been studied (fig. 1).
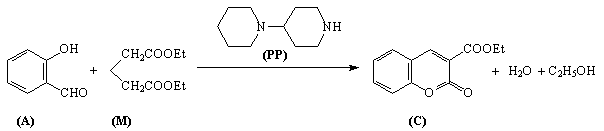
To improve consistency of experiments other catalyst was applied. The 4-piperidinopiperidine is soluble in non-polar solvents (e.g. toluene), non hygroscopic, air resist and crystalline solid in room temperature (mp 64-66°C).
The rate of disappearance of diethylmalonate is expressed by equation:
![]()
![]()

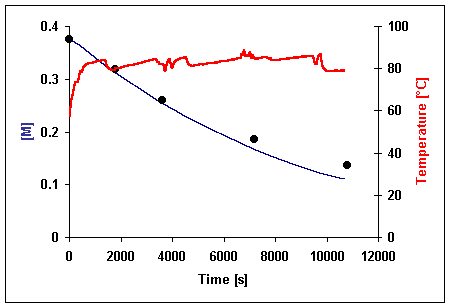
The agreement between the calculated and experimental concentrations shows that is no specific microwave effect on the investigated reaction (fig. 2 and 3).
Presented results does not confirm existence of non-thermal microwave effect in Knoevenagel condensation. Observed in previous [2] experiments reaction rate enhancement could be explain as influence of catalyst (piperidine) activity probably depended on trace amount of water.
[1] THE COMPARATIVE STUDY
OF THE KINETICS OF KNOEVENAGEL CONDENSATION UNDER MICROWAVE AND
CONVENTIONAL CONDITIONS, Szczepan Bednarz, Dariusz Bogdal, Fifth
International Electronic Conference on Synthetic Organic Chemistry
(ECSOC-5)
[2]
THE COMPARATIVE STUDY OF THE KINETICS OF KNOEVENAGEL CONDENSATION UNDER
MICROWAVE AND CONVENTIONAL CONDITIONS. Part II, Szczepan Bednarz, Dariusz
Bogdal, Sixth International Electronic Conference on Synthetic Organic
Chemistry (ECSOC-6)