[E009]
MICROWAVE ASSISTED EFFICIENT AND RAPID ONE-POT CONVERSIONS OF ALDEHYDES INTO NITRILES AND KETONES INTO AMIDES USING VARIOUS HETEROGENEOUS CATALYSTS
Biswanath Das*, K. V. N. S. Srinivas and P. Madhusudhan
INDIAN INSTITUTE OF CHEMICAL TECHNOLOGY, HYDERABAD-500 007, INDIA.
E-mail: [email protected]
Introduction
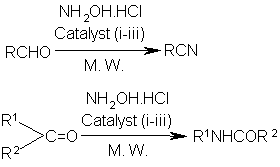
The conversions of aldehydes into nitriles1 and ketones into amides2 are important for the synthesis of various bioactive as well as industrially useful compounds. Nitriles are utilized for the preparation of anti-picornavirus and anti-inflammatory drugs and angiotension receptor ligands like losartan and valsartan.1 Amides are also used in medicine as febrifuge and the cyclic derivatives for the preparation of commercially useful polymeric compounds like Nylon 6.2 The transformations of nitriles to amines, amides, ketones, acids and esters and of amides to amines, acids and esters are of considerable synthetic importance. Nitriles can be prepared from aldehydes by dehydration of the corresponding aldoximes while amides from ketones by Beckmann rearrangement of the derived ketoximes. A limited number of microwave promoted one-pot conversions of aldehydes into nitriles and ketones into amides are also known but most of the methods suffer from certain drawbacks. One of the methods for conversion of aldehydes into nitriles under microwave irradiation utilized corrosive formic acid which may affect the sensitive aldehydes and the other method applied an oxidant, potassium peroxymonosulfate which may oxidise the functional groups.3 Mexican Bentonite which was employed for microwave assisted conversion of ketones into amides afforded very low yields of the products while hydroxylamine-O-sulfonic acid required tedious work-up procedure.4 Thus there is a need to develop improved methods for rapid one-pot conversions of aldehydes into nitriles and ketones into amides using efficient and easily available catalysts. Here we discuss our work on such conversions in the presence of three heterogeneous catalysts, silica supported sodium hydrogen sulfate (NaHSO4.SiO2),5 HY-zeolite6 and silica chloride7 under microwave irradiation.
Results and Discussion
Heterogeneous catalysts have recently gained importance for environmental and economic benefits. They can easily be handled and removed from the reaction mixture. Some of these catalysts can even be recycled. We have observed that three heterogeneous catalysts, NaHSO4.SiO2, HY-zeolite and silica chloride can efficiently be utilized for rapid one-pot conversions of aldehydes and ketones into nitriles and amides respectively by treatment with hydroxylamine hydrochloride (NH2OH.HCl) under microwave irradiation.

Catalyst: (i) NaHSO4.SiO2; (ii) HY-zeolite; (iii) silica chloride
The catalysts, NaHSO4.SiO2 and silica chloride can easily be prepared5,7 from the readily available ingredients, NaHSO4 and SOCl2 respectively and silica gel (finer than 200 mesh). The other catalyst, HY-zeolite is commercially available.
1. Conversion of aldehydes into nitriles
Several aryl and alkyl aldehydes on treatment with NH2OH.HCl under microwave irradiation in the presence of NaHSO4.SiO2 or HY-zeolite or silica chloride have been observed to undergo rapid conversion into the corresponding nitriles in high yields (Table 1). The conversion took place efficiently without using any solvent. Various functional groups like hydroxyl, ether, nitro and methylenedioxy remained intact. The double bond present in the aldehyde was also unchanged.
The mechanism of the conversion could briefly be proposed as follows. The aldehydes were first converted into aldoximes by reaction with NH2OH.HCl and the aldoximes were subsequently underwent rapid dehydration to yield the corresponding nitriles. It was observed that the aldoximes when separately irradiated under microwave irradiation in the presence of an used catalyst rapidly produced nitriles.
The experimental procedure for the preparation of nitriles is simple. A mixture of an aldehyde (1 mmol) and NH2OH.HCl (1.3 mmol) was mixed thoroughly with a catalyst (NaHSO4.SiO2 or silica chloride, 100 mg or HY-zeolite, 25 mg). The mixture was irradiated in a microwave oven (BPL, BMO, 700T, 466 watt) to produce the nitrile. The conversion occurred in very short time (Table 1). The catalyst used in the conversion can easily be separated by shaking the reaction mixture with CHCl3 or EtOAc followed by filtration. The product was isolated from the concentrated filtrate.
The catalytic activity of the three catalysts, NaHSO4.SiO2, HY-zeolite and silica chloride was almost similar in terms of the reaction time and yields of the products. HY-zeolite was found to be reused at least three times without losing its activity. The required amount of this catalyst was also less.
2. Conversion of ketones into amides
Various ketones were converted into the corresponding amides by treatment with NH2OH.HCl under microwave irradiation in the presence of a catalyst (NaHSO4.SiO2 or HY-zeolite or silica chloride) without using any solvent (Table 2). The conversion occurred rapidly with high yields of the products. Cyclohexanone was transformed to caprolactum, an industrially important compound. Various functionalities like alkyl, hydroxyl, ether and halogen could tolerate the reaction conditions.
The mechanism of the above conversion could be proposed as follows. The ketones first reacted with NH2OH.HCl to form ketoximes which then underwent Beckmann rearrangement to form the amides. The major products were produced by migration of the aryl groups of the intermediate ketoximes during rearrangement and the amides formed by alkyls migration were the minor products (yield < 5%).
In a typical experimental procedure a mixture of a ketone (1 mmol), NH2OH.HCl (1.5 mmol) and a catalyst (NaHSO4.SiO2 or silica chloride, 100mg or HY-zeolite, 25 mg) was irradiated inside a microwave oven. After completion of the reaction the mixture was shaken with CHCl3 or EtOAc and the catalyst was removed by filtration. The concentrated filtrate yielded the product.
The reaction time and yields of the products were almost similar in the conversion using any of the catalysts, NaHSO4.SiO2 or HY-zeolite or silica chloride. However, HY-zeolite can be recycled and less amount of this catalyst is required.
Conclusion
In conclusion we have developed a novel, simple and efficient practical method for one-pot conversions of aldehydes into nitriles and ketones into amides by applying microwave irradiation without using any solvent in the presence of an inexpensive heterogeneous catalyst (NaHSO4.SiO2 or HY-zeolite or silica chloride). The combination of microwaves and heterogeneous catalysts has made the process highly convenient. The yields of the products are very high and the time required for the conversion is very short. One of the catalyst, HY-zeolite can be reused. The experimental procedure is simple and environmentally benign.
Acknowledgements
The authors thank UGC, CSIR and IICT, India for financial assistance. They are also thankful to N. Ravindranath, B. Venkataiah, E. B. Reddy and I. Mahender for their help and suggestions.
Table 1. Conversion of Aldehydes (RCHO) into Nitrile (RCN)*
|
Entry |
R |
Catalyst† |
Time (min) |
Isolated yield (%) |
|
1 |
C6H5 |
i |
2 |
84 |
|
|
|
ii |
1 |
94 |
|
|
|
iii |
1 |
92 |
|
2 |
4-(HO)-C6H4 |
i |
1 |
90 |
|
|
|
ii |
1 |
95 |
|
|
|
iii |
1.5 |
94 |
|
3 |
3-(MeO)-C6H4 |
i |
1 |
91 |
|
|
|
ii |
1 |
92 |
|
|
|
iii |
1 |
94 |
|
4 |
3-(MeO), 4-(HO)-C6H3 |
i |
1 |
96 |
|
|
|
ii |
1 |
95 |
|
|
|
iii |
1.5 |
96 |
|
5 |
3,4-(MeO)2-C6H3 |
i |
1 |
97 |
|
|
|
ii |
1 |
94 |
|
|
|
iii |
1 |
95 |
|
6 |
3,4-(CH2O2)-C6H3 |
i |
1 |
95 |
|
|
|
ii |
1 |
92 |
|
|
|
iii |
1 |
92 |
|
7 |
4-(NO2)-C6H4 |
i |
2 |
87 |
|
|
|
ii |
1 |
96 |
|
|
|
iii |
1.5 |
91 |
|
8 |
C6H5CH = CH |
i |
2 |
87 |
|
|
|
ii |
2 |
84 |
|
|
|
iii |
2 |
90 |
|
9 |
C7H15 |
i |
3 |
81 |
|
|
|
ii |
2 |
86 |
|
|
|
iii |
2 |
87 |
|
10 |
C9H19 |
i |
3 |
80 |
|
|
|
ii |
2 |
87 |
|
|
|
iii |
2 |
89 |
* The structures of the products were established from their spectral (IR,
1H NMR and MS) and analytical data.
† Catalyst: (i) NaHSO4.SiO2; (ii) HY-Zeolite; (iii) silica chloride
Table 2. Conversion of Ketones (R1COR2) into Amides (R1NHCOR2)*
|
Entry |
R1 |
R2 |
Catalyst† |
Time (min) |
Isolated yield (%) |
|
1 |
C6H5 |
Me |
i |
2 |
91 |
|
|
|
|
ii |
2 |
94 |
|
|
|
|
iii |
3 |
90 |
|
2 |
C6H5 |
C6H5 |
i |
3 |
96 |
|
|
|
|
ii |
2.5 |
95 |
|
|
|
|
iii |
3 |
93 |
|
3 |
4-(Me)-C6H4 |
Me |
i |
2.5 |
93 |
|
|
|
|
ii |
2 |
94 |
|
|
|
|
iii |
2 |
91 |
|
4 |
4-(MeO)-C6H4 |
Me |
i |
2.5 |
90 |
|
|
|
|
ii |
2 |
95 |
|
|
|
|
iii |
2 |
93 |
|
5 |
4-(Br)-C6H4 |
Me |
i |
2.5 |
93 |
|
|
|
|
ii |
2.5 |
86 |
|
|
|
|
iii |
3 |
86 |
|
6 |
4-(Cl)-C6H4 |
Me |
i |
2.5 |
91 |
|
|
|
|
ii |
2.5 |
92 |
|
|
|
|
iii |
3 |
89 |
|
7 |
-(CH2)5- |
|
i |
3 |
85 |
|
|
|
|
ii |
2 |
95 |
|
|
|
|
iii |
2.5 |
91 |
* The structures of the products were established from their spectral (IR,
1H NMR and MS) and analytical data.
† Catalyst: (i) NaHSO4.SiO2; (ii) HY-Zeolite; (iii) silica chloride
References
1. (a) Diana, G. D., Cutcliffe, D., Volkots, D. L., Mallamo, J. P., Bailey, T. R., Vescio, N., Oglesley, R. C., Nitz, T. J., Wetzel, J., Girandu, V., Pevear, D. C. and Dutko, F. J., J. Med. Chem., 36, 3240 (1993). (b) Khanna, I. K., Weier, R. M., Yu, Y., Xu, X. D., Koszyk, F. J., Callins, P. W., Kobaldt, C. M., Veenhuizen, A. W., Perkins, W. E., Casler, J. J., Masferrer, J. L., Zhung, Y. Y., Gregory, S. A., Seibert, K. and Isakson, P. C., J. Med. Chem., 40, 1634 (1997).
2. (a) Finar, I. L. in “Organic Chemistry, Vol 1: The fundamental Principles” 6 th Ed., ELBS and Longman Group Ltd., London, P. 658 (1973). (b) Olah, G. A. and Fung, A. P., Synthesis, 537 (1979).
3. (a) Feng, J.-C., Lin, G., Dia, L. and Bian, N.-S., Synth. Commun. 28, 3765 (1998). (b) Bose, D. S. and Narasaiah, A. V., Tetrahedron Lett., 39, 6533 (1998).
4. (a) Delgado, F., Cano, A. C., Garcia, O., Alvarado, J., Velasco, L., Alvarez, C. and Rudler, H., Synth. Commun., 22, 2125 (1992). (b) Laurent, A., Jacquault, P., Di Martino, J.-L. and Hamelin, J., J. Chem. Soc., Chem. Commun., 1101 (1995).
5. (a) Das, B., Madhusudhan, P. and Venkataiah, B., Synlett, 1569 (1999). (b) Das, B., Ravindranath, N., Venkataiah, B. and Madhusudhan, P., J. Chem. Res (S), 482 (2000).
6. Srinivas, K. V. N. S., Reddy. E. B. and Das, B., Synlett, 625 (2002).
7. Srinivas, K. V. N. S., Mahender, I. and Das, B., Chem. Lett., 32, 738 (2003).