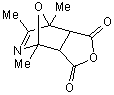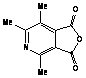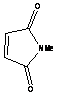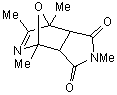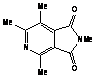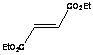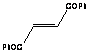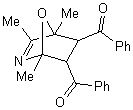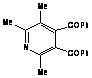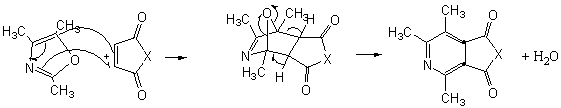7th International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30
November 2003
[E011]
Microwave - Assisted Synthesis of
Substituted Pyridines and Furans
Yousef M.
Hijji* and Achelle A. Edwards
Chemistry Department,
Morgan
State
University, Baltimore MD
21251
[email protected]
ABSTRACT:
Substituted Pyridines and Furans
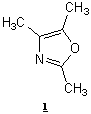
were synthesized from 2,4,5-Trimethyloxazole (diene), and olefinic and
acetylenic dienophiles by a microwave-assisted Diels-Alder reaction. The
reaction with olefinic dienophiles gave the adducts that underwent dehydration
under the microwave conditions to give substituted pyridines in good yields
within minutes. While the reaction with acetylenic dienophiles provided
substituted furan in excellent yield by Diels-Alder –retro Diels-Alder reaction.
Key Words:
Microwave, Oxazole, pyridine, Furan, Dies-Alder reaction, retro Diels-Alder,
olefinic dienophiles, acetylenic dienophiles.
INTRODUCTION:
The introduction of microwave energy into
conventional organic reactions, can decrease reaction times over 1000 fold and
thereby save both time and money. Another advantage of the reactions studied in
this paper is that they were carried out under neat conditions, in that no
solvent was used. Since these microwave driven reactions can work in a
solvent-less environment, this leads to less waste and improved synthetic
processing. The microwave has been used to carry out organometallic
cross-coupling reactions, heterocycles synthesis, nucleophilic additions and
substitutions, condensations, as well as cycloadditions.1
For the purposes of this paper, we would focus on the cycloaddition reactions in
the microwave.
The Diels-Alder
reaction has proven to be a very useful and easy method for the carbon-carbon
synthesis of cyclic and bicyclic organic compounds. It provides an efficient way
to synthesize six-membered carbon skeletons that maintain diverse
stereochemistry.
Oxazoles
have been used as dienes in the Diels Alder reaction.2,3,4 The
cycloaddition occurs at high temperature. The reaction with bis trimethylsilyl
acetylene gave the 3,4-disilylfuran by the Diels-Alder retro Diels Alder
reaction and loss of hydrogen cyanide. The process is very useful in providing
substituted furans.5 as the synthesis of 3,4-substituted is tricky
and challenging. A recent synthesis of furan dicarboxylate required few steps.6
With the developments of microwave application in organic synthesis, the
Diels-Alder reaction of oxazoles is a suitable example for the study of cyclo-addition
with acetylenic and olefinic dienophiles
2,4,5-Trimethyloxazole was the diene used in our study along with six different
dienophiles. In this study the reaction conditions were investigated and
products identified to explore it as a possible efficient method for the
synthesis of furans and pyridines.
EXPERIMENTAL:
All of the reactions were carried out in a
conventional microwave. The reactants were placed into a 4mL glass vial in the
absence of solvents, using a 1:1 mole ratio and the vial and its contents were
allowed to react in the microwave for a pre-selected amount of time. Also
because one of the aims of this experiment is to show a decrease in the reaction
time using the microwave, all of the reactions had to be performed using the
reflux in toluene, to serve as a comparison. Then using thin layer
chromatography (TLC), silica gel column chromatography and the Gas
chromatography-mass spectrometer (GC-MS), the products were separated, purified
and identified.
RESULTS:
Table1:
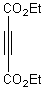
Diels-Alder reaction of 2,4,5-trimethyl oxazoles with acetylenic and olefinic
olefinic dienophiles under microwave irradiation to give furans and pyridines
respectively.
|
DIENE |
DIENOPHILE |
MICROWAVE |
ADDUCT
A |
SUBSTITUTED PRODUCT
B |
*YIELD |
|
 |
 |
4 min |
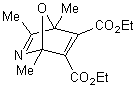 |
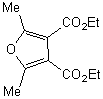 |
A. 6.25%
B. 93.75% |
|
 |
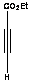 |
10.5
min |
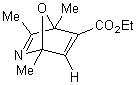 |
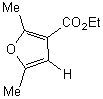 |
A. 47.1%
B. 52.9% |
|
1 |
3.
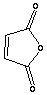 |
30 sec |
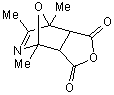 |
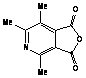 |
A. very low
B. 59.7% |
|
1 |
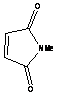 |
10.5
min |
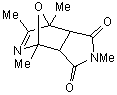 |
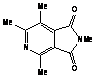 |
A. 43.0%
B. 57.0% |
|
1 |
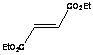 |
24 min
w. CeCl3 + silica gel |
No reaction |
No reaction |
______ |
|
1 |
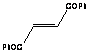 |
13 min |
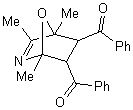 |
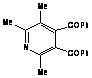 |
A. 68.7%
B. 31.3% |
Table2:
Diels-Alder reaction of 2,4,5-trimethyl oxazoles with acetylenic and olefinic
olefinic dienophiles under reflux in toluene to give furans and pyridines
respectively
|
DIENE |
DIENOPHILE |
REFLUX |
SUBSTITUTED
PRODUCT |
YIELD |
|
 |
 |
overnight |
 |
13.7%
|
|
1 |
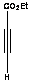 |
overnight |
 |
71.34%
|
|
1 |
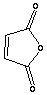 |
overnight |
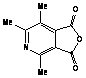 |
17.58% |
|
1 |
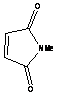 |
overnight |
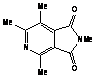 |
69.3% |
|
1 |
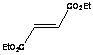 |
overnight |
No reaction |
______ |
|
1 |
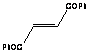 |
overnight |
No reaction |
______ |
DISCUSSION:
The reaction of 2,4,5-trimethyloxazole with
acetylenic dienophile tends to produce the adduct. This adduct proceed to lose
acetonitrile giving tri and tetra substituted furan in excellent yields more
than 90%, within minutes of microwave irradiation. The adduct and the furan can
be observed in the products too. This provides an efficient method for the
synthesis 3,- and 3,4- furan dicarboxylate. The synthesis of these compounds
requires many steps. The synthesis of 3,4-furan in general is a challenge and
tricky. The compounds can be produced in low after reflux in toluene for
overnight.
The more interesting is the reaction of
2,4,5-trimethyloxazole with acetylenic dienophiles as shown in table 1 is the
formation of the adducts that tends to dehydrate under the reaction condition to
give tetra and penta substituted pyridine. Such compounds are versatile
intermediates for the synthesis pyridine based alkaloids. The yields
were good, and the method is efficient as one pot synthesis of pyridine within
10 minutes. Table 2 depicts the reaction of 2,4,5-trimethyloxazole with variety
of dienophiles. The reactivity with maleic anhydride was high due to the high
reactivity of the dienophile and it was accomplished in seconds. Similar
products were obtained upon long heating under reflux, but in lower yields.
Diethylfumarate was unreactive for both microwave irradiated or refluxed
reactions while dibenzolyethylene was effective under the microwave conditions
while no product was obtained under reflux.
The mechanism for the reactions is presented in
schemes 1 and 2.
Scheme 1

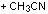
Scheme 2
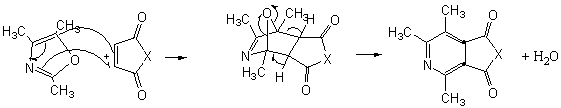
In conclusion this work demonstrate that
substituted furans and pyridines can be synthesized efficiently from oxazoles
under microwave conditions within minutes.
Acknowledgement:
We would like to thank the
NIH for support through Research Infrastructure in Minority Institution (RIMI)
grant # 5P20RR011606-02 and
NSF for Grant # HBCU-Rise- 0236753
References:
-
For
recent books on microwaves:
-
Hayes, B. L.; Microwave synthesis, Chemistry
at the speed of light, CEM publishing, 2002.
-
Loupy, A. Microwaves in organic synthesis,
Wiley VCH, Weinheim, 2003.
-
Kingston, H. M., Haswell,
Stephen J.” Microwave –Enhanced Chemistry, Fundamentals, sample preparation
and applications, American Chemical society, Washington D. C., 1997.
-
Keay, B. A.; Chem. Soc. Rev. 209-215 and
references therein.
-
Hou, X.; Cheung, H. Y.; Hon, T. Y.; Kwan, P.
L.; Lo, T. H.; Tong, S. Y.; Wong, H. N. C.; Tetrahedron, 1998, 54, 1955-2020
and references therein.
-
Wong, M. K.; Leung, C. Y.; and Wong, H. N. C.;
Tetrahedron, 1997, 53, 3497-351.
-
Hijji, Y. M.; Wanene J.; Obot, E.; Fuller,
J.; The fifth electronic conference in synthetic organic chemistry, E0023.
September 1-30, 2001.
www.mdpi.net/ecsoc-5.
-
Deshpande, A. M.; Natu, A. A.; Argade, N. P.;
Synthesis, 2002, 8, 1010-1014.

![]()
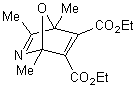


![]()
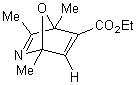

![]()