
7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[A007]
chemistry of substituted quinolinones. part 8.
synthesis and cyclization reactions of ethyl 5-amino-1-(1-methyl-2-oxoquinolin-4-yl)-3-methylsulfanylpyrazole-4-carboxylate *
Mohamed Abass
Department of Chemistry, Faculty of Education, Ain Shams University, Roxy, Cairo 11711, Egypt
Correspondence to M. Abass, E-mail: [email protected]
*This work is dedicated to the late Prof. Dr. Ahmed Mostafa Zahra
————————————————————————————————————————————————————————————
Abstract: The synthesis of the titled amino-ester 3 is described and its hydrolysis and chloro-acetylation led to the acid 5 and acetamide 7, which were cyclized to the pyrazolo-pyridones 6 and 8, respectively. Condensation of 3 with 2,5-dimethoxytetrahydrofuran afforded the pyrrolylpyrazole 9, which underwent cyclization by action of PPA to give pyrazolopyrrolizine 10. Treating 3 with thiophosgene gave the pyrazolyl isothiocyanate 11, which added aniline to yield the thiourea derivative 12, and cyclized to give pyrazolo-pyrimidinethiones 13-15. Condensation of 3 with formamide furnished pyrazolopyrimidine 16, whilst with triethyl orthoformate produced the ethoxymethyleneaminopyrazole 18, which condensed with hydrazine to give the aminopyrazoloprimidine 19. Reaction of 3 with Lawesson’s reagent resulted in the pyrazolothiazaphosphinine 21. Also the cyclization reaction of the compound 3 with malononitrile and its mixtures with carbon disulfide, or phenyl isothiocyanate, or benzaldehyde led to the formation of a variety of polyfunctional substituted pyrazolopyrimidines 23 and 26, pyrazolothiazine 24 and pyrazolopyridine 28.
Keywords: pyrazolylquinolinone, pyrazolo-azines, pyrazolo-azoles, cycliaztion reactions
—————————————————————————————————————————————————————————————
Studies on the biochemical properties of the structures bearing the pyrazole nucleus revealed their ability to uncouple oxidative phosphorylation, stabilize lysosomal membranes, inhibit biosynthesis of various mucopolysaccharides, and perhaps most importantly, inhibit prostaglandin biosynthesis, which accounts for their anti-inflammatory activities and analgesic effects [1]. Also several substituted pyrazolo[3,4-d]pyrimidines have shown diverse pharmacological activities, for example Allopurinol acts as a potent Xanthine-Oxidase (XO) inhibitor [2], and has been used as an effective therapy of both the primary hyperuricemia of gout and that secondary to haematological disorders or as antineoplastic therapy [3]. Unfortunately, Allopurinol has some deleterious side effects so that efforts to find novel potent and safe XO-inhibitors should be continued [4-6]. On the other hand, it is well known that 2(1H)quinolinone derivatives are associated with diverse pharmaceutical activities. Many quinolinones showed anti-hyperplastic activity [7], anti-cancer effects [8], Alzheimer’s disease healing action [9] and also anti-ulcer activity [10]. Due to the above-mentioned medicinal importance, it was planned to carry out a series of synthetic studies on novel heterocyclic derivatives those combined both pyrazole and quinolinone in one molecular-frame [11].
Results and Discussion:
Reactions of ketene dithioacetals with hydrazines are known to give the corresponding multi-functional pyrazole derivatives [12]. It is worthwhile mentioning that the heterocyclic products of these reactions are not only interesting from the viewpoint of biological applications, but also they have an important synthetic utility in the field of condensed heteropolycyclic compounds. Thence, 4-hydarzino-1-methyl-2(1H)quinolinone (1) was reacted with ethyl 2-cyano-3,3-bis(methylsulfanyl)acrylate (2) [13], in boiling DMF to afford ethyl 5-amino-1-(1-methyl-2-oxo-1,2-dihydroquinolin-4-yl)-3-methylsulfanyl-1H-pyrazole-4-carboxylate (3). 1H NMR spectrum of the amino-ester 3 showed the presence of a set of protons corresponding to an ethyl group as quartet and triplet peaks (J = 7 Hz) at d: 4.19 and 1.27 ppm, respectively. No indication was found for the formation of the other possible carbonitrile product 4. However, this result is coincident with the reported results of the reaction of compound 2 with hydrazines [12, 14] (Scheme 1).

Hydrolysis of the amino-ester 3 in an acid medium led to the formation of the corresponding amino-acid 5. De-esterification of compound 3 under either acidic or basic media was found to be not accompanied by replacement of either methylsulfanyl or amino groups. Eventually, acidic medium was selected for the higher yield. The preparation of the pyrazolopyridone 6 was accomplished from cyclization of the amino-acid 5 by means of acetic anhydride and glacial acetic acid [15]. Chloroacetylation of amino-ester 3 using chloroacetyl chloride furnished the corresponding chloroacetamide 7, in a good yield (86 %). Compound 7 was subjected to a cyclization reaction using triethylamine, in boiling DMSO, to give 5-chloro-4-hydroxy-3-methylsulfanylpyrazolo[3,4-b]pyridine 8, which is the chloro derivative of compound 6. Obviously, both compounds 6 and 8 sustain acidic character, as they are soluble in dilute alkalis and precipitated upon the addition of acids. Also both compounds 6 and 8 gave deep violet coloration with ferric chloride neutral solution due to the presence of phenolic hydroxyl group. Condensation reaction of the amino-ester 3 with 2,5-dimethoxytetrahydrofuran, in glacial acetic acid, led to the formation of the expected structure [16]; ethyl 5-pyrrolylpyrazole-4-carboxylate 9. The structure of the hetero-tricyclic derivative 9 was inferred from its correct elemental analysis as well as 1H NMR and IR spectra, which showed the disappearance of the amino group at the same time as a pyrrolyl moiety specific protons come into sight. An interesting linear hetero-polycyclic system bearing quinolinone was obtained, on carrying out catalyzed intramolecular cyclization, by action of polyphosphoric acid (PPA) on compound 9. The structure of the product was established on the basis of its spectral data. 1H NMR spectrum clearly showed that ethyl ester group disappeared and only seven aromatic protons were integrated in the spectrum chart, d: 7.83-7.25 ppm, beside the singlet peak at d: 6.96 ppm due to the proton at position-3 of quinolinone and also two singlets at d: 3.66 and 2.35 ppm due to both N-methyl and S-methyl protons, respectively. Building on the above cited data and other analyses, the structure of this product was deduced as 1-quinolinyl-3-methylsulfanylpyrazolo[4,3-b]pyrrolizin-4-one 10 (Scheme 2).

Isothiocyanates are distinguished as very useful intermediates for the preparation of thioureas, thiosemicarbazides, thioxopyrimidines, etc. Hence our attention was attracted to transform the amino group in the compound 3 to an isothiocyanato one. To reach this purpose, thiophosgene17 was used to obtain the targeted isothiocyanate 11 in a moderate yield. IR spectrum of compound 11 reflected the existence of the isothiocyanato group (n: 2129 cm-1). Addition of aniline to compound 11, in the molar ratio (1:1), furnished the 1,3-disubstituted thiourea 12, which underwent smooth base-catalyzed intramolecular cyclo-condensation to give 5-phenyl-6-thioxopyrazolo[3,4-d]pyrimidine 13. As another route to obtain compound 13, the amino-ester 3 itself was cyclized via an addition-condensation reaction with phenyl isothiocyanate, in the presence of ethanolic potassium hydroxide. It is thought that the formation of the pyrimidine 13 intermediately passes by the formation of the thiourea 12. Similar behavior was observed here again when compound 3 was treated with benzoyl isothiocyanate, under the same conditions, giving the thioxopyrazolo[3,4-d]pyrimidinone 14. The spectral data of the product showed that debenzoylation took place during the course of the cyclization reaction. However some reports in the literature recorded similar deacylation during the cyclization of acyl isothiocyanate and/or isoseleno-cyanate [18,19]. 5-Amino-3-methylsulfanyl-6-thioxopyrazolo[3,4-d]pyrimidinone 15 was obtained, in a relatively moderate yield (48 %), via a facile one pot preparation starting with amino-ester 3. Thus, compound 3 was reacted with carbon disulfide and potassium hydroxide, then the anion that formed was S-methylated by means of methyl iodide and subsequently the process was completed by in situ treatment with hydrazine hydrate. On the other hand, compound 15 was obtained through an easier method but in a lesser overall yield. This was achieved by cyclization reaction of the isothiocyanate 11 with hydrazine hydrate in boiling DMF (Scheme 3).

Thermal condensation of the amino-ester 3 with formamide led to smooth formation of pyrazolo[3,4-d]pyrimidinone 16. Compound 16 is another derivative of the pyrazolo-pyrimidine series, which is structurally related to Allopurinol2. Condensation of compound 3 with triethyl orthoformate was carried out, in boiling acetic anhydride, to afford the ethoxy-methyleneamino-ester 18. Although, the position-3 of 2-quinolinone has good susceptibility to be attacked by electrophiles [20] and it was expected in the latter reaction to undergo condensation with ethoxymethyleneamino group, affording pyrazolopyrimidoquinoline 17. Under different reaction conditions, the obtained product was only the compound 18. This may back to the geometry of the compound that is mainly expected to possess trans-form. Herein, no more than one product was isolated, which has a sharp melting point, checked with TLC and its spectral data pointed to a single and pure product. This was strongly supported by the cyclo-condensation of compound 18 with hydrazine hydrate in boiling DMF [21], the reaction that led to the formation of 5-amino-3-methylsulfanylpyrazolo[3,4-d]pyrimidin-4-one 19. The structural formula of compound 19 was established upon spectral and analytical data. The reaction of the amino-ester 3 with 2,4-bis(4-methoxy-phenyl)-1,3-dithia-2,4-diphosphetane-2,4-disulfide, Lawesson`s reagent, was carried out, in dry p-xylene, giving the novel pyrazolo[3,4-d][1,3,2]thiazaphosphinine 21. The spectral study of the product showed the disappearance of both the amino and carboxylate functions. It is worthwhile to assert that Lawesson’s reagent did not lead to the quinoline-thione 20 as all features of the product did not point to such replacement. In addition, such cyclization reactions, involving Lawesson’s reagent, were reported, leading to various phospha-heterocycles [22, 23] (Scheme 4).

The chemical behavior of the amino-ester 3 towards malononitrile was investigated. Thus, treating compound 3 with malononitrile, in the presence of sodium ethoxide, was carried out at the purpose of production of the pyrazolopyridine 22. The expected product 22 which should include amino and cyano groups was not afforded, while a different compound was obtained and characterized as (pyrazolo[3,4-d]pyrimidinyl)acetonitrile 23. Clearly, the inseparable intermediate; N-pyrazolylcyanoacetamidine was formed at first and hence the amino group underwent an intramolecular cyclo-condensation reaction with its neighboring carboxylate group. When the amino-ester 3 was reacted with malononitrile and carbon disulfide under solid-liquid phase transfer catalysis (PTC) conditions [24], (K2CO3/ [Bu4N]Br/ dioxane), we have obtained the (pyrazolothiazinylidene)-malononitrile 24. The production of the latter thiazine derivative might pass through the formation of a dicyanoketene-N,S-acetal intermediate (not separated), which in a following step underwent catalyzed cyclo-condensation between sulfanyl and carboxylate groups (Scheme 5).

Utilizing the same PTC conditions for the reaction of malononitrile and carbon disulfide with the starting compound, malononitrile and phenyl isothiocyanate was reacted with compound 3. As a very good support for the above hypothesis about formation of an intermediate ketene-acetal in such reactions, the dicyanoketene-N,N-acetal 25 was separated as the product of the latter reaction. The ketene-acetal 25 was well characterized through its analytical and spectral data, which confirmed the existence of both (NH) and ethyl carboxylate groups. Moreover, the compound 25 underwent smooth thermal cyclization to give the (pyrazolo[3,4-d]pyrimidin-ylidene)malononitrile 26. 1H NMR of the compound 26 gave us useful information about the nature of protons in this molecule, thus only one proton has the acidic character and appeared at d: 11.27 ppm due to (NH) of pyrimidinone, besides the other S-methyl, N-methyl and aromatic protons chemical shifts.
The reaction of compound 3 with malononitrile in the presence of a third partner reagent was once more performed with benzaldehyde under the same PTC conditions described here before. The reaction is expected to proceed via addition of the amino group in the amino-ester compound, to the so formed in situ; benzylidenemalononitrile, and subsequently an intramolecular nucleophilic cyclo-condensation took place between the dicyano-carbanion and the carboxylate group.
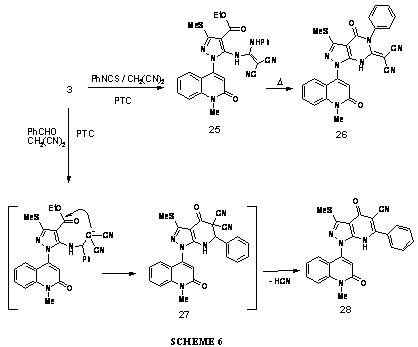
The compound 27 is thought to be an intermediate in this transformation, even though it was not isolated. The spectral data along with analytical results are well matched with the anticipated structure of the product pyrazolo[3,4-b]-pyridine-5-carbonitrile 28. This indicated that during the course of cyclization reaction one molecule of hydrogen cyanide should be expelled. As an evidence for the proposed structure for compound 28, we carried out an independent synthesis of this compound by action of benzoylacetonitrile on the same starting material 3, in the presence of sodium ethoxide as the catalyst (Scheme 6). All of the newly obtained compounds were characterized using analytical data as well as IR and 1H NMR spectroscopy.
[1] R. F. Borne, (Editors, W. O. Foye, T. L. Lemke and D. A. Williams), Principles of Medicinal Chemistry, Williams and Wilkins, Baltimore, p. 550 (1995).
[2] S. Kabashi, Chem. Pharm. Bull., 21, 941 (1973).
[3] G. W. Smith and V. Wright, Br. J. Clin. Prac., 41, 710 (1987).
[4] K. R. Hande, R. M. Noome and W. Stone, J. Am. J. Med., 76, 47 (1984).
[5] F. Arellano and J. A. Sacristan, Annals Pharmacother., 27, 337 (1993).
[6] Y. Osada, M. Tsuchimoto, H. Fukushima, K. Takahashi, S. Kondo, M. Hasegawa and K. Komoriya, Eur. J. Pharm., 241, 183 (1993).
[7] Y. Koga, Y. Kihara, M. Okada, Y. Inoue, S. Tochizama, K. Taga, K. Tachibana, Y. Kimura, T. Nishi and H. Hidaka, Bioorg. Med. Chem. Lett., 8, 1471 (1998).
[8] H. Yokozaki, R. Ito, S. Ono, K. Hayashi and E. Tahara, Cancer Lett., 140, 121 (1999).
[9] D. M. Fing, G. M. Bores, R. C. Effland, F. P. Huger, B. E. Kurys, D. K. Rush and D. E. Selk, J. Med. Chem., 39, 364 (1995).
[10] K. Otsubo, S. Morita, M. Uchida, K. Yamasaki, T. Kanbe and T. Shimizu, Chem. Pharm. Bull. (Tokyo), 39, 2906 (1991).
[11] M. Abass, Synth. Commun., 30, 2735 (2000).
[12] Y. Tominaga, Y. Honkawa, M. Hara and A. Hosomi, J. Heterocycl. Chem., 27, 775 (1990).
[13] L. Dalgaard, H. Kolind-Andresen and O.-S. Lawesson, Tetrahedron, 29, 2077 (1973).
[14] M. A. Raslan, R. M. Abd El-Aal, M. E. Hassan, N. A. Ahmed and K. U. Sadek, J. Chin. Chem. Soc., 48, 91 (2001).
[15] R. E. Lutz, J. F. Codington, R. J. Rowlett, A. J. Deinet and P. S. Bailey, J. Am. Chem. Soc., 68, 1810 (1946).
[16] M. C. De Sevricourt, H. S. El-Kashef, S. Raoult and M. Robba, Synthesis, 9, 710 (1981).
[17] M. Modica, M. Santagati, F. Russo, C. Selvaggini, A. Cagnotto and T. Mennini, Eur. J. Med. Chem., 35, 677 (2000).
[18] J. Sibor, D. Zurek, R. Marek, M. Kuty, O. Humpa, J. Marek and P. Pazdera, Collect. Czech. Chem Commun., 64, 1673 (1999).
[19] P. Pazdera, D. Ondracek and E. Novacek, Chem. Papers, 43, 771 (1989).
[20] E. A. Mohamed, M. M. Ismail, Y. Gabr and M. Abass, Chem. Papers, 48, 285 (1994).
[21] W. Ried, R. Guryn and J. Laoutidis, Liebigs Ann. Chem., 819 (1990).
[22] B. S. Pederesen and O.-S. Lawesson, Tetrahedron, 35, 2433 (1979).
[23] H. M. Moustafa, Phosphorus, Sulfur and Silicon, 164, 11 (2000).
[24] A. K. El-Shafei, H. Abdel-Ghany, A. A. Sultan and A. M. M. El-Saghier, Phosphorus, Sulfur and Silicon, 73, 17 (1992).