7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[A013]
A Novel Aromatic Iodination Method,
with Sodium Periodate Used Alone as the Iodinating Reagent
Chair and Laboratory of Organic Chemistry, Faculty of Pharmacy, Medical University,
PL 02-097 Warsaw, Poland, 1 Banacha Street
Tel./Fax: +(4822)5720643; E-mail: [email protected]
______________________________________________________________________________________________________________________
Abstract: Powdered NaIO4 suspended in anhydrous AcOH/Ac2O solvent mixtures acidified with concd H2SO4 (a catalyst and reagent) give a strong iodinating mixture, forming readily the new C-I bond in the reacted arenes, ArH. By pouring the final reaction mixtures into excess aq. Na2SO3 solution (a reductor), the crude iodoarenes, ArI, were isolated in high yields; after their purification, pure ArI were obtained in 45-86% yields.
Keywords: arenes, iodoarenes, aromatic iodination, sodium periodate, periodyl organic intermediates
______________________________________________________________________________________________________________________
Only inorganic derivatives of iodine(VII) are known. Few attempts to synthesize periodylarenes, ArIO3, ended off in failure. Up to now no single organoiodine(VII) compound has been synthesized and investigated [1, 2]. Willgerodt [3] had oxidized iodylbenzene, PhIO2, with a hot 30% solution of perchloric acid to obtain the expected periodylbenzene (,,Superjodobenzol,, or ,,Phenyljodtrioxyd,, according to his naming), PhIO3, but he instead obtained some white explosive crystals, probably an aromatic iodonium salt, Ph2I+ClO4-. Lewitt and Iglesias [4] had attempted to prepare PhIO3 by adding benzene dropwise to a chilled solution of H5IO6 in concd H2SO4, and reported to only obtain a 48% yield of periodobenzene, C6I6; they however observed that the colorless initial H5IO6/concd H2SO4 solution, after adding there the benzene, turned green then red, and finally light yellow, as the yellow-tan C6I6 gradually precipitated out. They remarked that the green intermediate first formed (presumably PhIO3) and next the red one should be further studied, and invited any investigator interested in this unusual reaction to pursue their research. Mattern [5] improved the above protocol for preparing either C6I6 or C6H2I4 from benzene, but he endeavored no effort to study the green and red intermediates observed visually by Levitt and Iglesias [4]. In contrast, perchloryl aromatics, ArClO3, have been known since 1958 [6]; they may detonate by vigorous shock or at higher temperatures. ArClO3 are comparatively stable compounds in acidic and neutral media, but hydrolize to phenols in strong alkali: ArClO3 + NaOH ® ArOH + NaClO3. They are unusually resistant to reduction, being inert even to such reagents as SnCl2 and hydrochloric acid, zinc and hydrochloric acid, hydrogen with palladium catalyst, LiAlH4 in diethyl ether, and acidified KI solutions. ArClO3 are thus less susceptible to reduction than perchloryl fluoride, FClO3, used for preparing ArClO3 from aromatic compounds, ArH, in the presence of AlCl3 (a Friedel-Crafts type of reaction). To the best of our knowledge, nobody has succeeded to prepare and investigate any single organobromine(VII) compound.
As to further continue our systematic studies on effective aromatic iodination reactions, which are related and explained in our two latest reviews [7, 8], we have recently decided to use alone sodium periodate, NaIO4, as the iodinating reagent. The reactions were carried out in anhydrous acetic acid/acetic anhydride solvent mixtures containing a chosen arene (Table), and then strongly acidified with varied quantities (see Experimental) of concd sulfuric acid. We have expected that the following subsequent reactions would proceed in the reaction mixtures, viz.
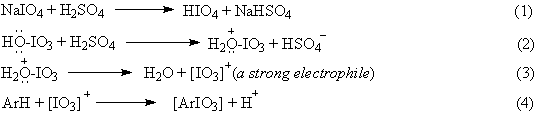
where: [IO3]+, hypothetical transient periodyl cations; [ArIO3], not isolable (vide infra), hypothetical periodyl intermediates of unknown stability.
The reaction mixtures were stirred at room temperature (not exceeding 35 oC) for 2 hours, then the temperature of the reaction mixtures was slowly increased (within 30-40 min) to 60-70 oC, and the mixtures were finally stirred at this temperature for a further 40-50 min (at higher temperatures, the evolution of the iodine vapors and the appearance of some crystals were observed). During the reactions we did not observe any green or red transient colorations. After cooling, the final reaction mixtures were poured into ice-water containing prior dissolved excess Na2SO3 (a reductor) to obtain the expected iodoarenes, ArI, in high crude yields, viz.

The oily or solid ArI were isolated in usual ways (see Experimental), and next they were purified to afford pure ArI in 45-86% yields (Table). Their purities and homogeneities were checked with TLC and satisfactory microanalyses (%I). Their chemical structures were supported with 1H NMR solution spectra and melting/boiling points close to those reported in the literature (Table). Also the unchanged mixed melting points with authentic specimens further supported the expected chemical structures.
We have to admit that in spite of our attempts, we could not isolate from the final reaction mixtures any ArIO3 intermediates. We shall try to achieve this aim otherwise.
In spite of our failure to isolate the expected (and still hypothetical) periodyl intermediates, ArIO3, we however succeeded to devise a novel protocol for the effective preparation of a number of monoiodoarenes, ArI, from the corresponding reacted arenes, ArH (Table). Transient meta-periodic acid, HIO4 (formed from NaIO4 in anhydrous and strongly acidic solutions, Eq. 1) was there the sole iodinating reagent, apt to readily form the stable C-I bond in the starting arenes. Further studies are in progress to extend the scope of our novel aromatic iodination method, and to throw more light on a possible mechanism of this reaction.
All the reagents and solvents were commercial (Aldrich) and were used without purification. The melting/boiling points of pure ArI (Table) are uncorrected. Elemental microanalyses (%I) were performed at the Institute of Organic Chemistry, the Polish Academy of Sciences in Warsaw. 1H NMR spectra were recorded at the Chair and Laboratory of Physical Chemistry, Medical University of Warsaw, at room temperature, with a Brucker 400 MHz spectrometer in CDCl3 solutions, and with TMS added as an internal standard.
Optimized Preparations of Iodoarenes from Arenes
Powdered NaIO4 (3.51 g, 16.4 mmol; 2.5% excess; only for PhBr and PhI: 3.42 g, 16 mmol; 0% excess) was suspended with stirring in a mixture made of glacial AcOH (15 mL) with Ac2O (10 mL)[only for 4-MeC6H4COOH: 20 mL AcOH + 15 mL Ac2O] cooled to 10 oC. A chosen arene (16 mmol, 0% excess; only for PhBr and PhI: 16.8 mmol; 5% excess) was added dropwise or portionwise. Still keeping the temperature at ca 10 oC, a given volume (see below) of concd (95%) H2SO4 was slowly added dropwise with stirring, viz.
a) for PhBr and PhI: 4.26 mL (7.85 g; 80 mmol) of concd H2SO4 was added;
b) for PhCOOH, PhCOOMe, and 4-MeC6H4COOH: 6.40 mL (11.8 g; 120 mmol) of concd H2SO4 was added;
c) for PhCOOEt and 4-ClC6H4COOH: 7.46 mL (13.7 g; 140 mmol) of concd H2SO4 was added;
d) for PhCF3: 8.53 mL (15.7 g; 160 mmol) of concd H2SO4 was added.
The reaction mixtures thus obtained were stirred at room temperature (not exceeding 35 oC) for 2 hours, then the temperature of the reaction mixtures was slowly increased (within 30-40 min) to 60-70 oC, and the mixtures were finally stirred at this temperature for a further 40-50 min. After cooling to r.t., the final reaction mixtures were poured into stirred ice-water (150 g) containing prior dissolved Na2SO3 (1.5 g). The oily crude products were extracted with CHCl3 (3 x 20 mL), the combined extracts were dried over anh. MgSO4, filtered, the solvent was distilled off, and the oily residues were fractionated under reduced pressure. The solid crude products were collected by filtration, washed well with cold water, air-dried in the dark, and recrystallized from appropriate organic solvents to afford the purified ArI (Table). The yields given for pure products were calculated from the total amounts of those reagents (ArH or NaIO4) which were used in the reactions in strictly stoichiometric quantities (0% excess).
|
Substrate |
Product |
Yielda (%) |
Mp [oC]/Sb or bp [oC/Tr] |
Lit. [9] mp [oC] or bp [oC/Tr] |
|
PhBr |
4-BrC6H4I |
58 |
89-91/EW(4:1) |
92 |
|
PhI |
1,4-I2C6H4 |
58 |
126-128/EW(4:1) |
129 |
4-(MeCONH)C6H4COOH |
3-I-4-(MeCONH)C6H3COOH |
79 |
242-243/EW(2:3) |
230 |
|
PhCOOH |
3-IC6H4COOH |
78 |
190-191/C |
188 |
|
PhCOOMe |
3-IC6H4COOMe |
75 |
46-48/EW(1:1) |
52 |
|
PhCOOEt |
3-IC6H4COOEt |
57 |
bp 151-154/36 |
bp 150.5/15 |
|
4-MeC6H4COOH |
3-I-4-MeC6H3COOH |
86 |
210-212/C |
210-212 |
|
4-ClC6H4COOH |
3-I-4-ClC6H3COOH |
60 |
214-216/EW(3:2) |
216-217 |
|
PhCF3 |
3-IC6H4CF3 |
45 |
bp 70-72/40 |
bp 84-88/40 |
a Yields of the purified products. Satisfactory microanalyses were obtained: I ± 0.3%.
b Solvents (S) used for recrystallization: C, CCl4; EW, ethanol-water.
1. (a) Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; VCH: Weinheim, 1992; (b) Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: San Diego, 1997.
2. Zhdankin, V. V.; Stang, P. J. in Chemistry of Hypervalent Compounds; Akiba, K., Ed.; Wiley-VCH: New York, 1999, Chap. 11, p. 349.
3. Willgerodt, C. Die organischen Verbindungen mit mehrwertigem Jod; Enke Verlag: Stuttgart, 1914, p. 6.
4. Levitt, L. S.; Iglesias, R. J. Org. Chem. 1982, 47, 4470.
5. Mattern, D. L. J. Org. Chem. 1983, 48, 4772-4773.
6. Inman, C. E.; Oesterling, R. E.; Tyczkowski, E. A. J. Am. Chem. Soc., 1958, 80, 5286-5288.
7. Skulski, L. Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland (1990-2000); Molecules, 2000, 5, 1331-1371. Avail. at URL: http://www.mdpi.org/molecules/papers/51201331.pdf
8. Skulski, L. Novel Easy Preparations of Some Aromatic Iodine(I, III, and V) Reagents, Widely Applied in Modern Organic Synthesis; Molecules, 2003, 8, 45-52. Avail. at URL: http://www.mdpi.org/molecules/papers/80100045.pdf
9. Dictionary of Organic Compounds; 6th ed.; Chapman & Hall: London, 1996.