7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[A020]
Thermal reaction of Tetracyclone (2,3,4,5-Tetraphenylcyclopentadienone) and its Derivatives with Molecular OxygenThies Thiemann,a* Shuntaro Matakaa, Jesus Iniesta Valcarelb, and David Waltonb
aInstitute for Materials Chemistry and Engineering, Kyushu University, 6-1, Kasuga-koh-en, Kasuga-shi, Fukuoka 816-8580, Japan
bSchool of Science and the Environment, Coventry University, Priory Street, Coventry, CV1 5FB, UK
Due to their characteristic, conjungated p-system and their relatively easy accessibility, tetraphenylcyclopentadienone (tetracyclone) (1a) and its derivatives have generated strong interest over the last 70 years.1 The redox behaviour of this class of molecules has been studied in some detail, although the reductive behaviour of the molecules leading to its radical anion is much better known than the oxidative behaviour. In particular, while there are numerous reports on the electrochemical reduction of the systems, there is no report on the direct preparative electrooxidation of tetracyclones.2 Our interest in an electrochemical oxidation of these compounds, especially in view of a comparative analysis to the electrooxidative behaviour of the related tetraarylthiophene S-oxides,3 led us to a preliminary study of the behaviour of tetracyclones towards oxygen under non-photolytic conditions. These experiments were also prompted by our identification of oxidation products of tetracyclone (tetraphenylcyclopentadienone) when the molecule was reacted with sterically hindered steroidal dienophiles in Diels Alder reactions at high temperatures.4
The reactions of tetracyclone (1a) and its derivatives with a number of oxidants have been reported, such as with peroxides, especially with hydrogen peroxide (in pyridine5a or acetic acid/acetic anhydride5b reaction systems),5 with peracids,6 and with nitric acid.7 Also, tetracyclone (1a) has been reacted with iodosylbenzene (PhIO) and cat. tetraphenylporphinato manganese (III) chloride (TPPMnCl),8 with bis(trimethylsilyl) monoperoxysulfate,9 and with chromiumpentoxide etherate as well as with chromium trioxide.10 Furthermore, tetracyclone (1a) has been reacted with superoxide anion.11
Additionally, the reaction of tetracyclone (1a) with photochemically12 and chemically13 generated singlet oxygen has been studied in detail.
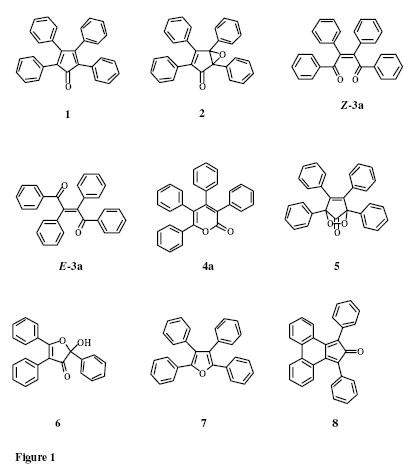
The oxidation of tetracyclone (1a) and of similar cyclopentadienones can follow a number of pathways, depending on the oxidant. The most important routes, though, are an initial epoxidation of the tetracyclone as is witnessed in the case of the reaction of tetracyclone with H2O25 and with PhIO and cat. TPPMnCl.6 The epoxide has been isolated, but it has also been shown that there are routes from the epoxide to the tetraphenyl-a-pyrone. A Baeyer Villiger type rearrangement leading from the tetracyclone (1a) to the a-pyrone 4a has been observed in the reaction with bis(trimethylsilyl) monoperoxysulfate.9 Also, the oxidation of tetra-aryltetracyclones with cerium ammonium nitrate (CAN)14 in aq. 75% THF has been reported to yield the lactones directly and to give tetra-aryl-a-pyrones 4 in fair yield. Different products, namely 2-hydroxyfuran-3-one 6 and tetraphenylfuran (7), are obtained by the reaction of tetracyclone with superoxide, where the reaction is thought to be initiated by a one-electron transfer from the superoxide to tetracyclone (1a) with subsequent addition of oxygen to the tetracyclone radical anion and follow-up reactions.11
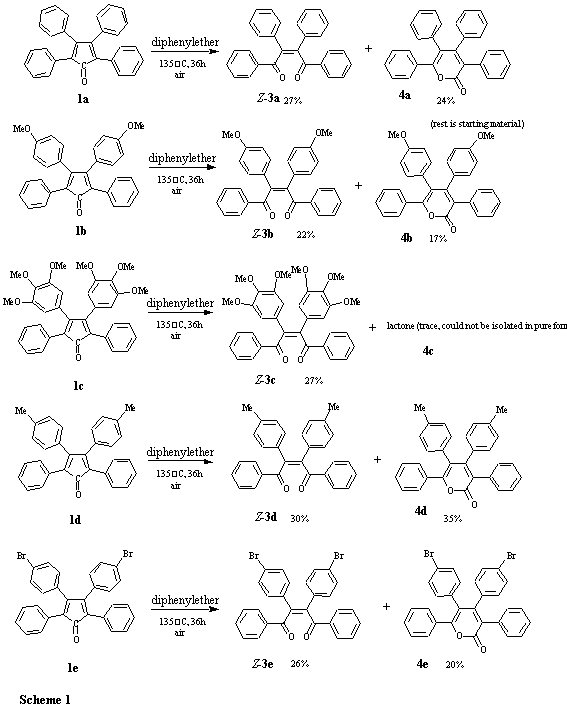
The photoirradiation of tetracyclone in the presence of oxygen is thought to proceed via an epidioxide 5, stemming from a 1,4-cycloaddition of singlet oxygen to the tetracyclone (1a). The epidioxide 5 then loses carbon monoxide to form (Z)-diacylstilbenes, Z-3a, which under the photolytic conditions can partly isomerize to the (E)-diacylstilbenes, E-3a. Thus, the photoreaction of also of other substituted tetracyclones in diethyl ether is known to give (Z)-a,a'-diacylstilbenes 3. Often, tetraphenyl substituted a-pyrone 4a is also formed in the reaction.
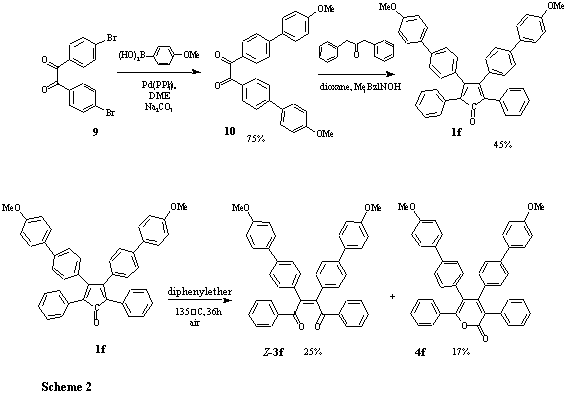
We have found that both (Z)-diacylstilbenes 3 as well as a-pyrones 4, being also products of most of the oxidation reactions of tetracyclone (1a) mentioned above, are formed in significant amounts, when tetracyclone or its derivatives are heated in a solvent that contains oxygen. Thus, when tetracyclone (1a) in diphenylether is heated at 135°C for 35h (under non-photolytic conditions), (Z)-1,2-dibenzoyl-1,2-diphenylethene (Z-3a) and tetraphenyl a-pyrone (4a) can be isolated in 27% and 24%, respectively, the remainder being unreacted starting material. A number of substituted tetraarylcyclopentadienones were subjected to the same procedure and again yielded the (Z)-diacylstilbene and a-pyrone, where the (Z)-diacylstilbene is in all cases the major product and where the a-pyrone is sometimes only present in trace amounts. To our knowledge, this thermal oxidative reaction of cyclopentadienones has only been commented upon once, namely in the work of W. Dilthey et al.,15 who have looked at the non-photolytic oxidation of phencyclone (8) in toluene and in pyridine. Nevertheless, the authors feel this oxidative 'degradation' of the cyclopentadienes is important to take into account when subjecting the molecules in cycloaddition reactions with sluggish dienophiles or to other reactions at higher temperatures in the presence of oxygen. Momentarily, study of the exact mechanism of the thermal oxidation of tetracyclone is in progress.
Experimental:
General:
Tetracyclone (1a) was obtained commercially as well as by known synthesis.16 Hexamethoxytetracyclone (1c) was prepared according to a procedure by K. Muellen et al.,17 which was slightly modified (dioxane, trimethylbenzylammonium hydroxide, 1h, reflux, 25% yield)
Melting points were measured on a Yanaco microscopic hotstage and are uncorrected. Infrared spectra were measured with JASCO IR-700 and Nippon Denshi JIR-AQ2OM machines. 1H- and 13C-NMR spectra were recorded with a JEOL EX-270 spectrometer. The chemical shifts are relative to TMS (solvent CDCl3, unless otherwise noted). Mass spectra were measured with a JMS-01-SG-2 spectrometer (EI, 70 eV). Column chromatography was carried out on Wakogel 300. All experiments leading to the preparation of the tetracyclones were purged with argon at the start.
Typical Experiment:
Unknown tetracyclone 1f was prepared from the commercially available p,p'-bis(bromophenyl)benzil (9) which was transformed to bis-(p-methoxybiphenyl)benzil (10) by Suzuki coupling (Scheme 2). The subsequent reaction of the benzil 10 with diphenylacetone was carried out by the usual procedure.
Preparation of tetracyclone derivative 1f: A deaerated mixture of commercially available p,p'-dibromobenzil (9) (700 mg, 1.90 mmol), p-methoxyphenylboronic acid (1.0 g, 6.58 mmol) and Pd(PPh3)4 (30 mg, 2.6.10-5 mol) in DME (6.0 mL) and 2M Na2CO3 (2.6 mL) was held at 70°C for 12h. Thereafter, the cooled reaction solution was diluted with water (10 mL) and extracted with chloroform (3 X 20 mL). The organic phase was dried and concentrated in vacuo. Column chromatography of the residue on silica gel gave p,p'-bis-(p-methoxyphenyl)benzil (10) (600 mg, 75%) as pale yellow needles; IR (KBr) n 1660, 1597, 1525, 1495, 1297, 1249, 1172, 1038, 883, 852, 818, 694 cm-1; 1H NMR (270 MHz, CDCl3) d 3.85 (s, 6H, 2 OCH3), 6.99 (d, 4H, 3J 8.6 Hz), 7.58 (d, 4H, 3J 8.6 Hz), 7.69 (d, 4H, 3J 8.2 Hz), 8.03 (d, 4H, 3J 8.2 Hz); 13C NMR (67.8 MHz, CDCl3) d 55.36 (OCH3), 114.50 (CH), 126.95 (CH), 128.50 (CH), 130.55 (CH), 131.18 (Cquat), 131.81 (Cquat), 147.12 (Cquat), 160.23 (Cquat), 194,18 (Cquat, C=O); MS (FAB, 3-nitrobenyl alcohol) m/z (%) 423 (MH+, 4), 211 (MeO-C6H4-C6H4CO+, 43). The reaction mixture was held at 65°C for 6h. Then the reaction mixture was diluted with chloroform (20 mL) and washed with water (2 X 10 mL). The organic phase was dried over anhydrous MgSO4 and concentrated. Column chromatography of the residue on silica gel (chloroform/ether/hexane 1 : 1 : 1 to chloroform) gave bis(p-methoxybiphenyl)diphenylcyclopentadienone (1f) as purple-brownish crystals, mp. 199°C; IR (KBr) n 2956, 2832, 1703, 1601, 1495, 1248, 1175, 1116, 1039, 828 cm-1; 1H NMR (270 MHz, CDCl3) d 3.84 (s, 6H, 2 OCH3), 6.95 (d, 4H, 3J 8.6 Hz), 7.01 (d, 4H, 3J 8.3 Hz), 7.40 (d, 4H, 3J 8.3 Hz), 7.52 (d, 4H, 3J 8.6 Hz); 13C NMR (67.8 MHz, CDCl3) d 55.38 (OCH3), 114.28 (CH), 125.41 (Cquat), 125.91 (CH), 127.44 (CH), 127.96 (CH), 128.08 (CH), 130.08 (CH), 130.22 (CH), 130.96 (Cquat), 131.30 (Cquat), 132.61 (Cquat), 140.63 (Cquat), 154.07 (Cquat), 159.48 (Cquat), 200.17 (Cquat, C=O). MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 597 (MH+, 9). HRMS Calcd. for C43H33O3: 597.2430. Found: 597.2432.
Thermal reaction of tetracyclone (1a) with oxygen: Tetracyclone (1a) (450 mg, 1.17 mmol) in diphenylether (2.0 mL) was heated at 135°C for 36h. Thereafter, the solution was subjected directly to column chromatography on silica gel (benzene) to give Z-3a (124 mg, 27%) and 4a (112 mg, 24%).
Selected spectroscopic and analytical data: Hexamethoxytetracyclone (1c), red-brown needles, mp. . - IR (KBr) n 3070, 2996, 2926, 1712, 1579, 1411, 1351, 1240, 1128, 1004, 852 cm-1; 1H NMR (270 MHz, CDCl3) d 3.45 (12H, s, 4 OCH3), 3.84 (s, 6H, 2 OCH3), 6.17 (s, 4H), 7.24 - 7.28 (m, 10H); 13C NMR (67.8 MHz, CDCl3) d 55.87, 61.01, 107.17, 125.26, 127.55, 128.09, 130.08, 130.76, 138.28, 152.56, 153.57, 199.95; MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 565 (MH+, 36), 564 (M+, 32). HRMS Found: 565.2228. Calcd. for C35H33O7: 565.2226 (MH+); Anal. Calcd. for C35H32O7: C, 74.45; H, 5.71%. Found: C, 74.07; H, 5.70%. 1,2-Dibenzoyl-1,2-bis(p-methoxyphenyl)ethene (Z-3b) as pale yellow needles. - mp. 189°C; IR (KBr) n 3058, 2926, 1657, 1605, 1509, 1250, 1175, 1031, 835, 692 cm-1; 1H NMR (270 MHz, CDCl3) d 3.74 (s, 6H, 2 OCH3), 6.71 (d, 4H, 3J 8.6 Hz), 7.08 (d, 4H, 3J 8.6 Hz), 7.41 (m, 2H), 7.28 (m, 4H), 7.83 (d, 4H, 3J 7.3 Hz); 13C NMR (67.8 MHz, CDCl3) d 55.13, 114.14, 127.74, 128.23, 129.97, 131.28, 132.74, 136.62, 143.47, 159.40, 197.39; MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 449 (MH+, 100). HRMS Found: 449.1756. Calcd. for C30H25O4: 449.1753; Anal. Calcd. for C30H24O (448.52): C, 80.33; H, 5.39%. Found: C, 80.48; H, 5.44%. 1,2-Dibenzoyl-1,2-bis(3',4',5'-trimethoxyphenyl)ethene (Z-3c) IR (KBr) n 3056, 2928, 1581, 1504, 1450, 1413, 1341, 1243, 1128, 1006, 750 cm-1; 1H NMR (270 MHz, CDCl3) d 3.59 (s, 12H, 4 OCH3), 3.79 (s, 6H, 2 OCH3), 6.40 (s, 4H), 7.26 - 7.48 (m, 6H), 7.87 (d, 4H, 3J 7.3 Hz); 13C NMR (67.8 MHz, CDCl3) d 56.12, 60.92, 107.04, 128.43, 129.85, 130.51, 133.21, 136.39, 138.25, 144.29, 153.30, 197.40; MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 569 (57). HRMS Found: 569.2174. Calcd. for C34H33O8: 569.2175. 1,2-Dibenzoyl-1,2-bis(p-bromophenyl)ethene (Z-3e)18,19 IR (KBr) n 2924, 1663, 1593, 1484, 1273, 1012, 829, 713 cm-1; 1H NMR (270 MHz, CDCl3) d 7.03 (d, 4H, 3J 8.2 Hz), 7.29 - 7.37 (m, 8H), 7.43 (m, 2H), 7.80 (d, 4H, 3J 8.2 Hz); d 13C NMR (67.8 MHz, CDCl3) d 123.07, 128.43, 129.96, 131.23, 132.16, 133.33, 133.82, 135.92, 143.79, 196.21 (C=O); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 559 ([81Br79Br]MH+, 12). HRMS Calcd. for C29H19O279Br81Br: 558.9734. Found: 558.9738 (MH+, FAB). 1,2-Dibenzoyl-1,2-bis(p-methoxybiphenyl)ethene (Z-3f): IR (KBr) n 3032, 2924, 1653, 1602, 1495, 1289, 1246, 1178, 1037, 826, 809 cm-1; 1H NMR (270 MHz, CDCl3) d 3.82 (s, 6H, 2 OCH3), 6.92 (d, 4H, 3J 8.9 Hz), 7.23 - 7.49 (m, 18H), 7.88 (d, 4H, 3J 7.2 Hz); 13C NMR (67.8 MHz, CDCl3) d 55.35 (OCH3), 114.25 (CH), 126.74 (CH), 127.96 (CH), 128.35 (CH), 130.10 (CH), 130.31 (CH), 132.47 (Cquat), 132.98 (CH), 133.58 (Cquat), 136.53 (Cquat), 140.50 (Cquat), 144.09 (Cquat), 159.45 (Cquat), 197.10 (Cquat, C=O). MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 601 (MH+, 38); HRMS Calcd. for C42H33O4: 601.2379. Found: 601.2377 (FAB, MH+). 4,5-Bis(p-bromophenyl)-3,6-diphenyl-a-pyrone (4e) IR (KBr) n 1717, 1485, 1072, 1011, 696 cm-1; 1H NMR (270 MHz, CDCl3) d 6.59 (d, 2H, 3J 8.6 Hz), 6.73 (d, 2H, 3J 8.5 Hz), 7.07 - 7.36 (m, 14H); 13C NMR (67.8 MHz, CDCl3) d 117.97, 121.83, 121.92, 125.50, 127.73, 127.96, 128.12, 128.32, 129.34, 129.81, 130.49, 130.91, 131.61, 132.24, 132.92, 133.62, 133.75, 134.79, 153.37, 157.45, 162.20; MS (70 eV) m/z (%) 548 ([81Br2]M+, 6), 546 ([79Br81Br]M+, 12), 544 ([79Br2]M+, 6), 105 (100). HRMS Calcd. for C28H18O279Br81Br: 545.9655. Found: 545.9658. 3,6-Diphenyl-4,5-bis(p-methoxyphenyl)-a-pyrone (4f) IR (KBr) n 2924, 1713, 1607, 1495, 1246, 826 cm-1; 1H NMR (270 MHz, CDCl3) d 3.80 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 6.78 (d, 2H, 3J 8.4 Hz), 6.86 - 6.94 (m, 6H), 7.15 - 7.45 (m, 18H); MS (FAB, 3-nitrobenzyl alcohol) m/z (%) 613 (MH+, 84), 584 (15), 479 (15). HRMS Calcd. for C43H33O4: Found: 613.2390 (MH+).
References and Footnotes:
1 M. A. Ogliaruso, M. G. Romanelli, E. I Becker, Chem. Rev. 1965, 65, 261.
2 Analytically, the electrochemical redox behaviour of tetracyclone has been studied to some degree, but no product analysis has been undertaken for the electrooxidative process: M. A. Fox, K. Campbell, G. Maier, L. H. Franz, J. Org. Chem. 1983, 48, 1762; T. Kawase, S. Ohsawa, T. Enomoto, M. Oda, Chem. Lett.1994, 1333.
3 D. J. Walton, J. Iniesta, M. Plattes, T. J. Mason, J. P. Lorimer, S. Ryley, S. S. Phull, A. Chyla, J. Heptinstall, T. Thiemann, H. Fujii, S. Mataka, Y. Tanaka, Ultrasonics Sonochemistry 2003, 10, 209.
4 T. Thiemann, M. Imai, unpublished results, Kyushu University, 2002.
5 5aJ. M. Dunston, P. Yates, Tetrahedron Lett. 1964, 505; 5bR. Pütter, W. Dilthey, J. Prakt. Chem. 1937, 149, 183.
6 D. C. Neckers, G. Hauck, J. Org. Chem. 1983, 48, 4691.
7 P. Yates, G. H. Stout, J. Am. Chem. Soc. 1954, 76, 5510.
8 T. Takata, R. Tajima, and W. Ando, Chem. Lett.1985, 665.
9 W. Adam, A. Rodriguez, J. Org. Chem. 1979, 44, 4969.
10 J. E. Baldwin, J. L. Swallow, H. W. S. Chan, J. Chem. Soc., Chem. Commun. 1971, 1407 and ref. cited.
11 11aA. A. Frimer, G. Strul, H. E. Gottlieb, J. Org. Chem. 1995, 60, 4521; 11bI. Rosenthal, A. A. Frimer, Tetrahedron Lett. 1975, 3731; 11cI. Rosenthal, A. A. Frimer, Tetrahedron Lett. 1976, 2805; 11dB. Pandey, M. P. Mahajan, R. K. Tikare, M. Muneer, N. P. Rath, P. V. Kamat, M. V. George, Res. Chem. Intermed.1991, 15, 271.
12 12aN. M. Bikales, E. I. Becker, J. Org. Chem. 1956, 21, 1405; 12bC. Dufraisse, A. Etienne, J. Aubry, Compt. Rend.1954, 239, 1170; 12cC. Dufraisse, A. Etienne, J. Aubry, Bull. Soc. Chim. 1954, 21, 1201; 12dG. O. Schenck, Z. Elektrochem.1952, 56, 855; 12eC. F. H. Allen, J. A. VanAllan, J. Org. Chem. 1953, 18, 882; 12fJ. Rigaudy, N. Kim Cuong, J. Baranne-Lafont, P. Duminy, C. Chassagnard, Tetrahedron 1986, 42, 1345.
13 13aJ.-M. Aubry, S. Bouttemy, J. Am. Chem. Soc. 1997, 119, 5286; 13bF. El Khatib, C. Tachon, A. M. Caminade, M. Koenig, Tetrahedron Lett. 1985, 26, 3007.
14 Tse Lok Ho, T. W. Hall, C. M. Wong, Synth. Commun.1973, 3, 79.
15 W. Dilthey, S. Henkels, M. Leonhard, J. Prakt. Chem. 1938, 151, 97.
16 J. R. Johnson and O. Grummitt, Org. Synth.1943, 23, 92.
17 F. Dötz, J. D. Brand, S. Ito, L. Gherghel, K. Müllen, J. Am. Chem. Soc. 2000, 122, 7707.
18 For Z-3d, see also: A.V. Vasil'ev, A. P. Rudenko, Russ. J. Org. Chem. 1997, 33, 1555; W. Ried, R. Lantsch, Chem. Ber. 1971, 104, 679.
19 Z-3e had been reported previously: J. Smidt, R. Sieber, Angew. Chem. 1959, 71, 626; M. Koral, E. I Becker, J. Org. Chem. 1962, 27, 1038.