

2-AMINOETHYL-NITRATES AS NEW AGENTs
FOR THE COMMON PROTECTION OF CARBONyl group


Jan IMBERA, Pavel PAZDERA
Abstract
New agent for protection of the carbonyl group into ketones, aldehydes and carboxylic acids incl. their functional derivatives via oxazolidines and oxazolines formation by an application of 2-aminoethyl-nitrates as the agent is described.
One of the possible and new synthetic applied method for the protection of carbonyl group is a reaction of carboxylic acids and their derivatives, aldehydes and ketones with 2-aminoethyl-nitrates resulting 2-substituted oxazolines (in the case of using of carboxylic acids and their functional derivatives) or 2-subtitutes oxazolidines (by using of ketones or aldehydes). Current methods for the protection of carbonyl group use 2-aminoalkohols or their derivatives like 2-aminohalides, etc. However, these manner of derivatization demand relatively high reaction temperatures, they are time consuming and for achievement of high yields is necessary the water removal, e. g. by an azeotrope evaporation. Condensation reactions need a present of high acid catalysts. They are unusable for some organic compounds, which are very sensitive to these reaction conditions (optically active nature compounds, etc.). Now we found that presented disadvantages are possible very simply well go round by an application of 2-aminonitrates. These can be prepared very simply from 2-aminoethanols or their derivatives by treatment with nitric acid.
2. New method for protection of carbonyl group by an application of 2-aminoethyl-nitrates as the agent
In this presentation we demonstrate on some examples the application of methodology for the protection of carboxylic acid functional derivatives, aldehydes and ketones, respectively. We should say that this is just the beginning for the proving of this agent to a whole range of carboxylic acid functional derivatives, aldehydes and ketones.
Scheme I
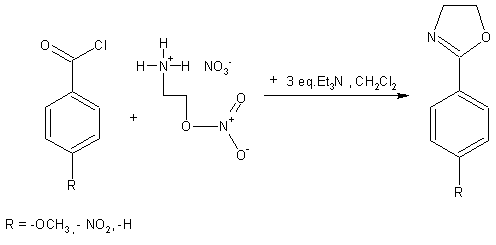
The yields of developed 2-substituted oxazolines were increased from benzoyl chloride to 4-nitrobenzoyl chloride in the range 75 - 95%. The resulting oxazolidines were sufficiently pure for further reactions.
General procedure:
Triethylamine (75 mmol) was slowly added to the suspension containing acyl chloride (25 mmol) and 2-aminoethyl nitrate salt (25 mmol) in 50 ml of dichloromethane under stirring on the ice bath.
After addition of all triethylamine the mixture was stirred at the ambient temperature about 1 h, then washed with water (2x20ml) and the organic layer was dried by anhydrous Na2SO4.
Filtration followed by evaporation of solvent afforded the crude product, which was distilled under reduced pressure or recrystalized from ethanol to give the pure compound.
Table I.
|
OXAZOLINE from acyl chlorides |
Reaction conditions |
Yields from salt of 2-aminoethylnitrate |
Yields (Lit.)from 2-aminoalkohols |
|
2-phenyloxazoline |
CH2Cl2, Et3N, R.T. 1 h |
75% |
30-50% (1, 2, 3, 5) |
|
4-methoxyphenyl-2-oxazoline |
CH2Cl2, Et3N, R.T. 1 h |
85% |
78 % (3, 4, 5) |
|
4-nitrophenyl-2-oxazoline |
CH2Cl2, Et3N, R.T. 1 h |
95% |
72% ( 2, 5 ) |
For the reaction on ketones as the starting educts we choose cyclohexanone and for aldehydes the benzaldehyde. In this case developed 2-substituted oxazolidines (Scheme II)
Scheme II.
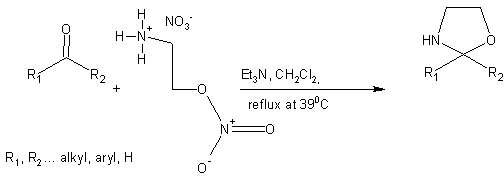
In both cases this reactions proceeded excellent with very good yields as it is demonstrated in Table II.
Table II.
|
OXAZOLIDINES from |
Reaction conditions |
Yields from salt of 2-aminoethylnitrate |
Yields (Lit.)from 2-aminoalkohols |
|
Cyclohexanone |
CH2Cl2, Et3N, R.T. 1 h |
95-98% |
89% (6,7) |
|
Benzaldehyde |
CH2Cl2, Et3N, R.T. 1 h |
90% |
78% (6) |
4. Discussion
In this method we choose for the first part of reaction study a simple available 2-aminoethanole. It shows that these cyclization reactions proceed very readily for all applied benzoyl chlorides to corresponding 2-substituted oxazolines. For all of these compounds the reaction conditions were very mild and the reaction time short. The analogical results we obtain by using benzaldehyde and cyclohexanone. From these results we come to conclusion that it will be very useful prove many substituted derivatives of carboxylic acids, aldehydes and ketones to react with 2-aminoethanol nitrates and substituted 2-aminoethanols under this mild conditions to produce 2-substituted oxazolines and oxazolidines, respectively, in very good yields.
It seems to be that these reactions proceed like very fast and these may bring convenient utilization for the protection reactions of many compounds in the organic synthesis.
1/ Wenker H., J. Amer. Chem. Soc. 57, 1079 (1935).
2/ Wiley R. H., Leonard L., Bennet J., J. Amer. Chem. Soc. 70, 447 (1948).
3/ Leffler M.T., Adams R., J. Amer. Chem. Soc. 59, 2252 (1937).
4/ Rehlander P., Chem. Ber. 27, 2154 (1894).
5/ Cwik A., Hell Z., Horwáth F., Tetrahedron Lett. 43, 3985 (2002).
6/ Badiang J. C., Aubé J., J. Org. Chem. 61, 2484 (1996).
7/ McNab A., Hamish J.: J. Chem. Soc. (Perkin. Trans.) 4, 548 (2002).