7th International Electronic
Conference on Synthetic Organic Chemistry (ECSOC-7),
http://www.mdpi.net/ecsoc-7, 1-30 November 2003
[A034]
3-Formylchromones
III. ♠
Synthetic, Kinetic and Theoretical Study of Novel Solvatochromic System
of 2-[(4H-4-Oxo-benzopyran-3-yl)ethenyl]benzothiazolium Salts
Pavol Kois 1*, Dusan Loos1, Jan
Lac 2, Anton Gaplovsky 3, Mario
Klestinec1 and Margita Lacova
1*
*Corresponding authors: [email protected], [email protected] Tel. +421 2 60296-338 or -341
♠Previous part 3-Formylchromones II - see ECSOC-7
Abstract: Synthesis of novel 2-(4H-4-oxo-benzopyran-3-yl)benzothiazolium salts is presented by fast and one-pot
condensation of 3-formylchromones with 3-benzyl-2-methylbenzothiazolium (or benzoxazolium) bromides, using classical conditions or
microwave irradiation. The product of solvolysis of
2-(4H-4-oxo-benzopyran-3-yl)benzothiazolium salts was
identified. The microwave irradiation showed a beneficial effect on these
reactions, especially in improving overall yields and purity of products.
Prepared compounds were identified by elemental analysis and by 1H
NMR spectra.
The color changes of prepared salts after interaction
with water, DMF, DMSO, methanol and CHCl3 were measured by UV and fluorescence
spectral kinetic methods.
Quantum-chemical AM1 method was used to study
all structural parameters, to perform full geometry optimization, to calculate
heats of formation and charge densities.
Keywords: Chromone,
3-Formylchromone, Benzoxazolium salt, Microwave
synthesis, AM1 Quantum-chemical method, , Aldol reaction, Kinetic
Introduction
This work is aimed
at the preparation of chromones bonding benzothiazolium or benzoxazolium
groups at C-3 position of chromone ring. As a substituents these azole salts have strong electron withdrawal properties. Generally, C-3 modified chromones are useful reactive agents. They are acceptors of
nucleophiles and they can rearrange under mild
conditions1-4. It
is obvious these salts have versatile synthetic utility in the field of new heterocycles.
In our earlier
papers we described the condensations of 3-formyl-chromones with various
heterocyclic components with active methyl- or methylene-
groups as creatinine5, rhodanine5, benzothiazoles6,
furopyroles7, butanolides8. None of these chromone derivatives showed any solvato-chromic
effect. Later we studied the photochemical behavior, photochromism
and thermo-chromism of carboxyimide
derivatives of chromone by UV and fluorescent
spectral methods9.
Benzothiazole salts are known as antihelmintic10,
antineoplastic11, and antimicrobial12,13 agents. It is worth mentioning that the
activity of various chromone salts on photosynthetic
electron transport in spinach chloroplast we also reported14, 15.
The main goal of
this work was a synthetic study of chromone salts 3 and 4 by classical method and by microwave-irradiation, and then the study of
sensitivity of these compounds to water by kinetic and theoretical methods.
Results and
Discussion
Synthetic study
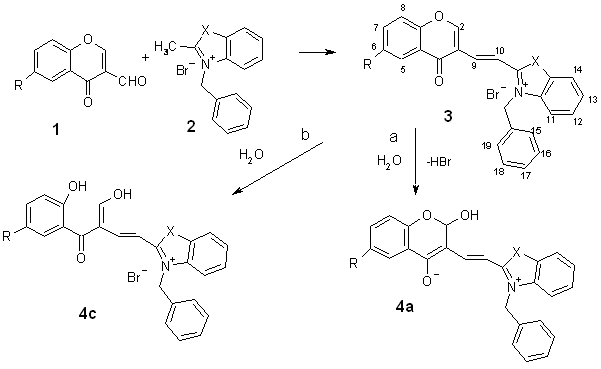
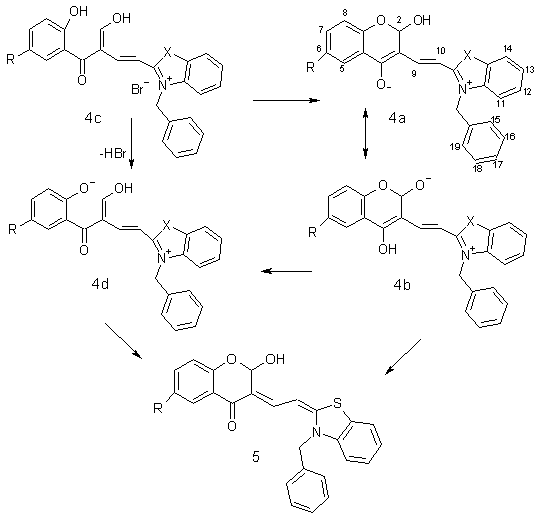
Scheme 1. R = H, Cl,
Br, CH3, NO2
X = S, O
We report here on the fast, facile
and one-pot synthesis of 2-[(4H-4-oxobenzopyran-3-yl)ethenyl]benzothiazolium
(or benzoxazolium) salts by condensation of three
starting compounds: 3-formylchromones, 2-methylbenzothiazole and benzylbromide in solvents CH3NO2, CH3CN,
CHCl3 or (CH3 CO)2O.
Experimental results showed that N-substituted
2-methylbenzothiazolium salt 2 as a starting material was more convenient
component for the condensation than 2-methylbenzoazoles6
in the classical as well as microwave-assisted aldol
synthesis.
The microwave
irradiation of the reaction mixture for 8-10 min gave yields about 10-30 %
higher with cleaner product in comparison with classical conditions (3-4 hours,
90-100 ºC). The products are pale yellow solids with high melting points,
rather insoluble in common solvents. Their color is sensitive to water – yellow
color can change to red, violet or brown. The rate of color changes is so
rapid, that this method can be used for determination of water content in some
solvents. Our explanation of the mechanism of nucleophilic
reaction at C-2 of compounds 3 in the
presence of traces of water in solvent is illustrated on Schemes 1 and 2
Reaction of 3 with water ends in the compounds 4 or 5. The detailed investigation of reaction mixture
by TLC, 1H NMR and UV spectroscopy excluded the presence of
compounds 5, but all experimental results
confirmed the presence of compound 4c.
This compound was prepared as a stable molecule after addition of water to acetonitrile solution of 3
and then irradiation by microwave.
Scheme 2. R = H, Cl, Br, CH3, NO2
X = S, O
Kinetic study
The solvatation
ability of prepared salts 3 is related
to their polarity and can play important role in the color changes. These salts
absorb UV-light in the range 200–420 nm in anhydrous solvents. Absorption
maximum is red shifted with increasing polarity of solvent (Fig.1).
DMF l1max=386 nm, l2max=490 nm;
DMSO l1max=386 nm, l2max=498 nm;
CHCl3 lmax=396 nm
This absorption corresponds to p, p*band, which overlaps n, p* band.
After addition of small amount of
water to the methanol solution of this compound there appears a new long-wave
absorption band in a visible
region (Fig.2 and Fig.4).
The intensity of new band is proportional to water concentration in solution.
At the same time the intensity of
bands in the range of l=
390 nm are proportionally going down (Fig.2 and Fig.3).
The kinetic study revealed a rapid
chemical equilibrium between compounds 3
and their water adducts 4 in methanol
solution. It showed a marked linear dependence of changes on the square of
water concentrations
of compounds 4 and their
adducts (Fig.3 and Fig.5).
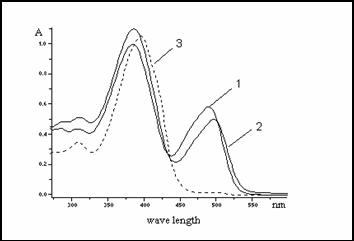
Fig.1.: Absorbance of
2-Benzyl-2-[2-(chromon-3-yl) ethenyl]benzothiazolium bromide in
1. DMF l1max=386 nm, l2max=490 nm
2.
DMSO l1max=386 nm, l2max=498 nm,
3.
CHCl3 lmax=396 nm
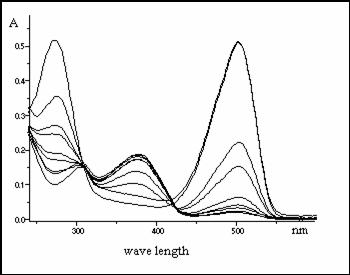 Fig.2.: Absorbance of 3-benzyl-2-[2-(chromon-3-yl)ethenyl] benzothiazolium bromide
in methanol
Fig.2.: Absorbance of 3-benzyl-2-[2-(chromon-3-yl)ethenyl] benzothiazolium bromide
in methanol
after gradual
addition of water (0.044, 0.088, 0.133, 0.177, 0.222, 0.266, 0.311, 0.355,
0.400 mol.dm-3)
Band at λmax. = 378 nm is decreasing
Band at λmax. = 502 nm is increasing
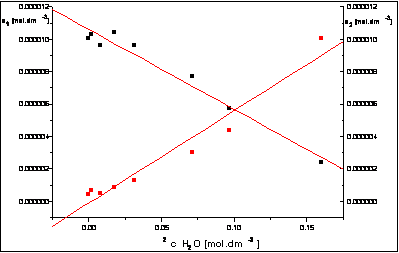 Fig.3.: The dependence of
concentration changes of benzothiazole derivatives 3 and 4 on square of water concentration in methanolic
solution of samples.
Fig.3.: The dependence of
concentration changes of benzothiazole derivatives 3 and 4 on square of water concentration in methanolic
solution of samples.
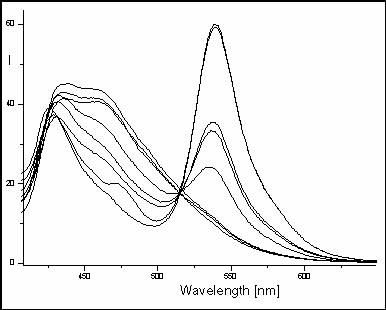 Fig.4: Fluorescencent
spectra
Fig.4: Fluorescencent
spectra
Effect of water concentration in
methanol solution of 3-benzyl-2-[2-(chromone-3-yl)ethenyl]benzothiazolium bromide
on the gradual decrease of emission band lexc =380nm and
simultaneous increasing of emission band at
lmax =540nm
(water concentration - 0.044, 0.088, 0.133, 0.177, 0.222,
0.266, 0.311, 0.355, 0.400 mol.dm-3)

Fig.5: Fluorescencent spectra
Effect of water
concentration in methanol solution of 3-benzyl-2-[2-(chromone-3-yl)ethenyl] benzothiazolium
bromide. The gradual increasing of fluorescent emission at band lexc =540
nm (water
concentration - 0.044, 0.088, 0.133,
0.177, 0.222, 0.266, 0.311, 0.355, 0.400 mol.dm-3)
Theoretical study
The optimal structures and quantum chemical
parameters of starting compounds and products 1-5 were calculeted
by the AM1
method16 with
standard parametrization and full optimization of all
geometrical parameters. Schemes 1 and 2 comprise our proposal of possible mechanism of
water addition on compounds 3.
From calculated values of heat of
formation of compounds 3 is evident, that reaction with benzothiazole salts is energetically preferable to benzoxazole salts. The electronwithdraving
substituent in benzothiazole
derivatives decreases the reaction ability, on the
contrary the reaction ability in the benzoxazole
derivatives is increasing.
According to the difference of heat
of formation the preferred reaction of compounds 3 hydratation
is (b) to compound 4c, in comparison to step (a) to compound 4a. It
means that opening of chromone ring occurs. Substituents in the position 6 of chromone
system and heteroatoms in benzoazole
ring have influence on corresponding reaction rates (Table 1).
According to heat of formation is
clear that benzoxazole derivatives should react
better than benzotiazole derivatives. Similar effect
has also the substituent with electronwithdrawing
character in position 6 (compound 3), which decreases bond order O1-C2,
very significantly especially in the case of benzoxazole
derivatives. The energy for the formation of compounds 4c is 240 kJ larger than for
compound 4a.
Geometry optimization for compound 4a allways resulted in compound 5 as is clear from calculated bond
orders and charge densities, as well as corresponding bond lengths. Otimization of compounds 4b geometry (#18 in Table 1)
was successful only for structure R=H. Substituents on 4b, (Cl or NO2)
decreased very significantly bond
order O1-C2 . For this reason
the opened structure of 4b is more stable and preferred.
For dehydrobromination
reaction of 4c
series, the difference of heat of formation (200 kJ.mol-1) prefers
the formation of compounds 4a (5), in contrast to 4d.
Table 1.
Thermodynamic
parameters for salts 3 and chromones 4, 5
|
No. |
Comp. |
R |
X |
Q(O1) |
Q(C2) |
P(O1-C2) |
DHf(kJ/mol) |
|
01 |
1 |
H |
- |
- |
- |
- |
-222.064 |
|
02 |
1 |
Cl |
- |
- |
- |
- |
-251.087 |
|
03 |
1 |
NO2 |
- |
- |
- |
- |
-199.735 |
|
04 |
2 |
- |
S |
- |
- |
- |
919.719 |
|
05 |
2 |
- |
O |
- |
- |
- |
842.723 |
|
06 |
3 |
H |
S |
-0.098 |
0.126 |
1.1595 |
825.553 |
|
07 |
3 |
Cl |
S |
-0.097 |
0.125 |
1.1550 |
805.084 |
|
08 |
3 |
NO2 |
S |
-0.099 |
0.115 |
1.1300 |
869.326 |
|
09 |
3 |
H |
O |
-0.093 |
0.147 |
1.1801 |
910.755 |
|
10 |
3 |
Cl |
O |
-0.092 |
0.146 |
1.1153 |
889.816 |
|
11 |
3 |
NO2 |
O |
-0.094 |
0.138 |
1.1502 |
952.376 |
|
12 |
4a (5) |
H |
S |
-0.226 |
0.201 |
0.9190 |
45.364 |
|
13 |
4a (5) |
Cl |
S |
-0.223 |
0.202 |
0.9150 |
17.576 |
|
14 |
4a (5) |
NO2 |
S |
-0.218 |
0.205 |
0.9003 |
59.210 |
|
15 |
4a (5) |
H |
O |
-0.226 |
0.202 |
0.9178 |
-50.091 |
|
16 |
4a (5) |
Cl |
O |
-0.224 |
0.203 |
0.9139 |
-77.950 |
|
17 |
4a (5) |
NO2 |
O |
-0.218 |
0.205 |
0.8992 |
-36.467 |
|
18 |
4b |
H |
S |
-0.335 |
0.268 |
0.0043 |
184.822 |
|
19 |
4c |
H |
S |
-0.274 |
0.139 |
0.0007 |
668.171 |
|
20 |
4c |
Cl |
S |
-0.269 |
0.135 |
0.0006 |
645.764 |
|
21 |
4c |
NO2 |
S |
-0.224 |
0.132 |
0.0002 |
683.537 |
|
22 |
4c |
H |
O |
-0.277 |
0.157 |
0.0009 |
584.293 |
|
23 |
4c |
Cl |
O |
-0.223 |
0.156 |
0.0006 |
562.384 |
|
24 |
4c |
NO2 |
O |
-0.223 |
0.151 |
0.0002 |
603.804 |
|
25 |
4d |
H |
S |
-0.556 |
0.119 |
0.0003 |
269.320 |
|
26 |
4d |
Cl |
S |
-0.540 |
0.124 |
0.0002 |
226.933 |
|
27 |
4d |
NO2 |
S |
-0.494 |
0.137 |
0.0001 |
226.837 |
|
28 |
4d |
H |
O |
-0.560 |
0.131 |
0.0003 |
175.866 |
|
29 |
4d |
Cl |
O |
-0.545 |
0.136 |
0.0003 |
134.145 |
|
30 |
4d |
NO2 |
O |
-0.499 |
0.149 |
0.0002 |
135.623 |
Experimental part
General
Melting points (uncorrected) of
synthesized compounds were measured on a Kofler
heated block. All compounds were analyzed for C, H, N, S.
The results obtained were within ±0.4% of theoretical values. 1H NMR
spectra (in d-scale, ppm) were recorded on the VARIAN GEMIN 2000, 300MHz
spectrometer in DMSO-d6. For some compounds, TESLA BS487 (80MHz) instrument
using saturated solutions of the compounds in CF3COOH-d was used. UV
VIS spectra were obtained in 1cm thick cell using the HP Diod
Array 8254 spectrophotometer, fluorescence spectra using the spectrofluorimeter Hitachi F-2000.
Kinetic experiments were measured until the limiting equilibrium concentration
was reached.
The
3-formylchromones 1 were prepared by Vilsmeier double formylation of
appropriate 2-hydroxyacetophenones17. The preparation of benzothiazolium salts by classical procedures was carried
out as described in detail elsewhere5.
All microwave
assisted reactions were carried out in a Lavis–1000 multiQuant microwave oven. The apparatus was equipped with
magnetic stirring and an external reflux condenser to suit the laboratory
applications.
3-Benzyl-2-methylbenzothiazolium bromide 2
A stirred mixture of
2-methylbenzothiazole (0.5 g, 3.35 mmol) and benzylbromide (0.573 g, 3.35 mmol)
in anhydrous nitromethane (2 ml), or acetonitrile (2 ml) was irradiated for 20 minutes at 270 W
in microwave oven. Pale-green precipitate was diluted by acetone, filtered off
and dried. Yields were about 55 % (acetonitrile) 70 %
(nitromethane), m.p. 241 -
243 °C. The product was identical to that prepared by classical method5,6 as evidenced by 1H NMR.
3-Benzyl-2-[(6-R-chromon-3-yl)ethenyl]benzothiazolium bromides 3
Method A (by irradiation).
A stirred mixture of
3-benzyl-2-methylbenzothiazolium bromide (1 mmol) and
3-formylchromone derivative (1 mmol) in 2 ml
anhydrous nitromethane was irradiated at 270 W for
2-8 minutes. Solid products were filtered off, washed with warm acetone and
crystallized from acetonitrile.
Method B (one - pot - reaction, by
irradiation).
A stirred mixture of benzylbromide (1 mmol),
2-methylbenzothiazole (1 mmol) and appropriate
3-formylchromone derivative (1 mmol) in anhydrous nitromethane (2 ml) was irradiated at 270 W for 2-8
minutes. The isolation of products was the same as for method A. Yields were in the range
84-89%.
Method C (classical conditions).
The same mixture as
in method A
was refluxed for 6 hours in argon atmosphere. The crystals were filtered off
and recrystallized from acetonitrile.
Yields were about 50-60 %.
Benzoxazole derivative 3g
was also prepared by the method B.
Table 2.
Reaction time, yields and melting points of chromones 3a - *3g (microwave irradiation)
|
compounds |
3a |
3b |
3c |
3d |
3e |
3f |
*3g |
|
reaction time [min.] |
10 |
8 |
6 |
7 |
4.5 |
6 |
2.5 |
|
yields [%] |
75.9 |
68.2 |
76 |
81 |
81 |
79 |
54 |
|
melting point o[C] |
215-217 |
171-173 |
242-245 |
253-256 |
294-297 |
255-258 |
221-223 |
*benzoxazole derivative
Table 3.
Analytical
characteristics of chromones 3
|
Comp. |
Formula Mr |
Elemental
analysis (calc. / found) |
|||||
|
%C |
%H |
%N |
%S |
%Br |
|||
|
3a |
H |
C25H18BrNO2S 476.4 |
63.03 63.21 |
3.81 3.86 |
2.94 2.82 |
6.73 6.35 |
16.77 17.06 |
|
3b |
CH3 |
C26H20BrNO2S 490.4 |
63.89 63.68 |
4.11 4.32 |
2.86 2.73 |
6.54 6.38 |
16.30 16.58 |
|
3c |
Cl |
C25H17BrClNO2S 510.8 |
58.78 58.25 |
3.35 3.42 |
2.74 2.61 |
6. 28 6.06 |
15.64 15.21 |
|
3d |
Br |
C25H17Br2NO2S 555.3 |
54.08 53.84 |
3.09 3.12 |
2.52 2.47 |
5.77 5.68 |
28.78 29.12 |
|
3e |
NO2 |
C25H17BrN2O4S 521.4 |
57.59 57.32 |
3.28 3.35 |
5.37 5.14 |
6.15 5.95 |
15.33 14.82 |
|
3f |
CH3 |
C26H19BrClNO2S 524.8 |
59.50 59.30 |
3.65 3.57 |
2.67 2.76 |
6.11 5.89 |
15.22 15.84 |
|
*3g |
H |
C25H18BrNO3 460.3 |
65.23 64.94 |
3.94 3.82 |
3.04 3.04 |
|
17.36 17.56 |
*Benzoxazole derivative
Preparation of compound 4
The
mixture as above (method A) was irradiated at 270 W for 10 minutes. Then water
was added (2 mmol) and the irradiation was prolonged for
another 20 minutes. After cooling, the red crystals were filtered off and
washed with warm acetone. The yield of compound 4(R=H)
with m.p. 220-223 °C was 60 %.
Elemental analysis for C25H19NO3S, Mr 413.5; (calc. 72.55 %C, 4.59%H, 3.38%N; found 72.42%C, 4,48%H, 3.21%N)
1HNMR ( DMSO-6d) d(ppm) : 3.28s(1H, 4-OH); 6.0s,(2H, CH2); 6.40s(1H,H-2); 7.30s(5H, H-Ph); 7.81 - 7.89m(4H, H-Btz.); 8.06d(1H, H-9, 3J=15Hz); 8.702 (d,1H, H-10, 3J=15Hz);
(CF3COOH-d):
6.15 (s, 2H); 7.39 (s, 5H, H-Ph); 7.61 (t, 1H, H-5); 7.81 - 7.89 (m, 4H, H-Bt-het.); 8.06 (d, 1H, H-9, 3J=15Hz); 8.702 (d, 1H,
H-10, 3J=15Hz); 8.19 (dd, 1H, H-6, 3J=8Hz,
4J=1.43Hz); 8.32 (d, 1H, H-8, 3J=8Hz); 8.50 (dd, 1h, H-7, 3J=8Hz, 4J=1.44Hz);
9.174 (s, 1H, H-2)
Table 4.
1H NMR data of compounds
3
|
Comp. |
1H NMR ,d(ppm
) , DMSO-d6, 300 MHz, 100 oC |
|
3a |
6.15s,( 2H, CH2); 7.39s,( 5H, Ph); 7.71t,( 1H, 3J=7.97Hz, 3J=7.14Hz, H-8); 7.77d, (1H, 3J=8.24Hz, H-11); 7.81-7.89 m,(3H,H-5,6,7); 8.11d ( 1H, 3J=15.66Hz, H-10); 8.21d,( 1H, 3J=.7.69Hz ,H-13); 8.31d ( 1H, 3J=8.24Hz, H-12); 8.59d, ( 1H, 3J=7.69Hz, H-14); 8.69d ( 1H, 3J=15.66Hz, H-9); 9.17 s, (1H, H-2) |
|
3b |
2.47 (s, 3H, CH3); 6.15 (s, 2H, CH2); 7.40 s,(5H, Ph); 7.71-7.72m, (2H, H-8,11); 7.8-7.89m, (2H, H-7,14); );7.96 d,4J=1.7Hz, H-5); 8.07d ( 1H, 3J=15.4Hz, H-10); 8.35d ( 1H, 3J=9.8Hz, H-12); 8.52d ( 1H, 3J=10Hz, H-11); 8.70d ( 1H, 3J=15.4Hz, H-9); 9.18s, ( 1H, 3J=15.38 Hz, H-10) |
|
**3c |
6.11 (s, 2H, CH2); 7.307-7.505 (m, 5H, Ph); 7.761-8.326 (m, 8H, H-5,7,8,10 -14); 8.744 (s, 1H, H-10); 9.008 (d, 3J=15.38 Hz, 1H, H-9) |
|
3d |
6.16 (s, 2H, CH2); 7.37-7.41 m,(5H, Ph); 7.79-7.88m, (3H, H-8,11,12); 8.01dd,(1H, 3J=7.2Hz , 4J=2.Hz, H-7); 8.06d, ( 1H, 3J=15.6Hz, H-10); 8.24d,(1H,4J=1.9Hz, H-5); 8.34d ( 1H, 3J=8Hz, H-13); 8.52d ( 1H, 3J=8Hz, H-14); 8.66d ( 1H, 3J=15.5Hz, H-9); 9.21s, ( 1H, H-2) |
|
3e |
6.21s,( 2H, CH2); 7.33-7.46 (m, 5H, Ph); 7.79-7.92 (m, 2H, H-12,13); 8.05d ( 1H, 3J=9.15Hz, H-8); 8.11 (d, 1H, 3J=.15Hz ,H-9); 8.37d ( 1H, 3J=8.24Hz, H-11); 8.59d, ( 1H, 3J=8.55Hz, H-14); 8.63dd ( 1H, 3J=9.15Hz, 4J= 2.9Hz,.H-7); 8.74 d ( 1H, 3J=.15Hz ,H-10); 8.8d ( 1H, 4J=.2.9Hz ,H-5); 9.36 s (1H, H-2) |
|
3f |
2.46 (s, 3H, CH3); 6.16 (s, 2H, CH2); 7.39 s,(5H, Ph); 7.79-7.89m, (2H, H-11,12); 7.91d, (1H, 4J=1.7Hz, H-7);7.94 d,(1H4J=1.7Hz, H-5); 8.05d ( 1H, 3J=15.7Hz, H-10); 8.36d ( 1H, 3J=8.6Hz, H-13); 8.52d ( 1H, 3J=8.7Hz, H-14); 8.67d ( 1H, 3J=15.7Hz, H-9); 9.24s, ( 1H, H-2) |
|
*3g |
5.98m s,( 2H, CH2); 7.36-7.86m ( 5H, H-15 - 19); 7.73-7.86m, ( 4H, H-6,8,12,13); 7.89-7.97m, (1H, H-7); 8.06d,(1H,3J 7.42Hz, H-14 ); 8.14d,(1H,3J 7.44Hz, H-11 ); 8.22dd,(1H,3 J 7.92Hz,4J=1.65Hz,H-5 ); 8.33d,(1H,3J 15.65Hz, H-10 ); 8.52d,(1H,3J 15.68Hz, H-9 ); 9.29s, 1H, H-2) |
*benzoxazole derivatives ** measured in CF3COOH-d, 80 MHz
Conclusions
The novel solvatochromic systems was developed by condensation of 3-formylchromones with 3-benzyl-2-methylbenzothiazolium (or benzoxazolium) bromide. Synthesis by microwave irradiation is safer and at times more convenient alternative to classical method. . Kinetic measurements and theoretical calculations of hydration reaction demonstrated new and interesting properties of our chromone derivatives. Several questions remain to be answered with respect to the reactivity of chromone-heterocyclic conjugates. Among them is the question of the reactivity of chromone conjugates with various nucleophiles, e.g. amino compounds. These experiments are underway.
Acknowledgements: Financial support for this research by the Slovak Grant Agency is
gratefully acknowledged, Grant No. 1/8207/01 and 1/0071/03
References
1. Sabitha,G., Aldrichimica Acta 1996, 29,
15.
2. Ghosh,
C.K.; Bandyopadhyay Ch., J. Chem. Soc. Perkin Trans. 1, 1983, 1989.
3. Haas,G.;
Stanton,J.,L.; Sprecher,A.; Wenk,P., J.Heterocyclic
Chem. 1981, 18, 607.
4. Stankovicova, H.; Lacova;M., Gaplovsky, A.; Chovancova,J.; Pronayova,N., Tetrahedron 2001, 57, 3455.
5. Gasparova, R.; Lacova,
M., Collect. Czech.
Chem. Commun. 1995, 60, 1178.
6. Gasparova, R.; Lacova,
M.; El-Shaaer, H.M.; Odlerova,
Z., Il Farmaco 1997, 52, 251.
7. Krutosikova,A.;
Lacova,M.; Dandarova, M.; Chovancova, J., ARKIVOC
2000, 2, 409.
8. Melikyan, G.,S.;
Lacova, M.; Kralova, K.;
El-Shaaer, H.,M.; Henselova, M.; Avetisyan,
A., A., Chem. Papers 1993, 47, 388.
9. Gaplovsky, A.; Donovalova,
J.; Lacova, M.; Mracnova,
R.; El-Shaaer,H.,M., Journal of Photochemistry and Photobiology,
A: Chemistry 2000, 136,
61.
10. Garmaise, D.L, Paris, G.Y., Komlossy, J., Chambers, C.H.: J. Med. Chem. 1968, 12, 30.
11. Krieg, M., Bilitz,
J.M.: Biochem. Pharmacol. 1996, 51,
1461
12. Magdolen, P., Zahradnik,
P., Foltinova, P.: Drug Res. 2000, 50(II),
1023
13. Buffa, R., Zahradnik,
P., Foltinova, P.: Heterocyclic Commun. 2001, 7, 331
14. Kralova, K.; Sersen,
F.; Lacova, M.; Stankovicova,
H. Biol. Plant. 1996,
38, 397.
15. Kralova, K.; Sersen,
F.; Gasparova R.; Lacova,
M., Chem. Papers 1998, 52, 776.
16. AMPAC 6.7, 2001 Semichem, 7128 Summit, Shawnee, KS 66216
17. Nohara, A.; Ishiguto, T.; Sanno, Y., Tetrahedron
1974, 13, 1183.