
8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. 1-30 November 2004. http://www.lugo.usc.es/~qoseijas/ECSOC-8/
Martin Dolezal*1, Lukas Palek1, Zuzana Roslerova1, Jiri Kunes2, Vladimir Buchta3, Katarina Kralova4
1 Department of Pharmaceutical Chemistry and Drug Control, 2 Department of Inorganic and Organic Chemistry, 3 Department of Biological and Medical Sciences, Faculty of Pharmacy, Charles University, Heyrovskeho 1203, 500 05 Hradec Kralove, Czech Republic
4 Institute of Chemistry, Faculty of Natural Sciences, Comenius University, Mlynska Dolina CH-2, 842 15 Bratislava, Slovak Republic
* Author to whom correspondence should be addressed;
e-mail: [email protected], tel.: +420-49-5067272, fax: +420-49-5512423
Abstract: Newly synthesised pyrazinamide analogues with carboxamide moiety as a bridging ligands between basic and aromatic areas of molecule were tested for their antifungal and photosynthesis-inhibiting activity. The synthetic approach, analytical and spectroscopic data of all newly synthesized compounds are presented. The highest antifungal effect (MIC < 62.5 µmol l-1) against Candida albicans, the most susceptible fungal strain tested, was found for 6-chloropyrazine-2-carboxylic acid (3-chlorophenyl)amide. The most active inhibitor of oxygen evolution rate in spinach chloroplasts was 5-tert-butyl-6-chloropyrazine-2-carboxylic acid (3-chlorophenyl)amide (IC50 = 47.0 µmol dm-3).
Keywords: Substituted amides of pyrazin-2-carboxylic acid; Antifungal evaluation; Photosynthesis inhibition; Spinach chloroplasts
Over the last three decades there has been a dramatic increase in the incidence of fungal infections [1]. Systemic candidiasis, aspergillosis, histoplasmosis and cryptococcosis constitute still serious and of growing importance problem in medicine. Fungal infections caused by endemic, dimorphic fungi, as well as by filamentous fungi, also have increased significantly in recent years [2]. Mycoses of epidermal skin (dermatomycosis), hair, nail (onychomycosis) and cornea mycoses (tineas) are the most frequent forms of fungal diseases.
Promising new compounds have recently been identified in an effort to supplement the relatively sparse portfolio of antifungal drugs. Many of these compounds have defined mechanisms of action against fungal cells and have, in some cases, aided the identification of new selective targets in fungi. For most of these compounds, however, factors such as a narrow spectrum of activity, susceptibility to efflux pumps, protein binding, serum inactivation and poor pharmaceutical properties prevent their use in the clinic. Even so, these compounds are novel substrates for synthetic modifications that could lead to the discovery of future antifungal drugs [3]. Also some pyrazine derivatives exert promising antifungal activity. Some 5-halo-2-methylanilides of 2,3-dimethyl-5-(N-alkylkarbamoyl)pyrazine-2-carboxylic acid were synthesized and tested for their preemergence fungicidal activities [4].
Various compounds possessing -CONH- moiety were found to inhibit photosynthetic electron transport [5]. Amides of 2-alkylpyridine-4-carboxylic [6,7], 2-alkylsulfanylpyridine-4-carboxylic [7,8] acids inhibited oxygen evolution rate in Chlorella vulgaris and their inhibitory activity depended on the lipophilicity of the compounds. Several esters of alkoxy substituted phenylcarbamic acids showed the antialgal activity against Chlorella vulgaris [9-11]. We have recently reported the synthesis of a series of amides prepared from the substituted pyrazine-2-carboxylic acids and some aminophenols [12], halogenated and alkylated anilines [13-16]. All these amides possess some antialgal, antifungal, and antimycobacterial properties [13,15,17].
The presented study is concerned in the synthesis of another series of amides prepared from substituted pyrazine-2-carboxylic acids and 4-fluoroaniline, 3-chloroaniline or 2-chloro-4-hydroxyaniline, respectively. The aim of this work is to search for the structure-activity relationships in the mentioned series, i.e. to continue in studying of the substituent variability influence on the biological activity, and to determine the importance of increased hydrophobic properties for antifungal and/or photosynthesis-inhibiting evaluation of newly prepared substituted pyrazine-2-carboxamides.
The synthesis of starting pyrazinecarboxylic acids 1a-d is shown in Scheme 1 [12,18-20]. The synthesis of amides 2a-l is shown in Scheme 2. Condensation of chlorides of pyrazine-2-carboxylic [18,19], 6-chloropyrazine-2-carboxylic [20], 5-tert-butylpyrazine-2-carboxylic [12] or 6-chloro-5-tert-butylpyrazine-2-carboxylic [12] acids with ring-substituted anilines yielded a series of 12 amides of pyrazine-2-carboxylic acids 2a-l. The melting points, yields, and the IR, 1H and 13C NMR spectral data for the all compounds prepared are given in Experimental. Calculated log P values of all derivatives studied are shown in Table 2.
The antifungal activity of 12 amides was investigated in vitro against Trichophyton mentagrophytes (TM), Candida albicans (CA), C. tropicalis (CT), C. krusei (CK), C. glabrata (CG), Trichosporon beigelii (TB), Aspergillus fumigatus (AF), and Absidia corymbifera (AC) by the broth micro dilution method (for results see Table 1). A moderate antifungal activity against T. mentagrophytes and C. albicans was found for several compounds. A significant activity against the other tested fungal strains was not observed. The most active amides in the series were pyrazine-2-carboxylic acid (4-fluorophenyl)amide (2a), and particularly 6-chloropyrazine-2-carboxylic acid (3-chlorophenyl)amide (2f). The compounds 2g and 2 h were completely inactive against all tested fungi.
Twelve studied compounds inhibited photosynthetic electron transport in spinach chloroplasts (see Table 2). The photosynthesis-inhibiting activity of the compounds was investigated as inhibition of oxygen evolution rate in spinach chloroplasts. The IC50 values (see Table 2) varied in the range from 47.0 (2g) to 722.0 µmol dm-3 (2i). The inhibitory activity of the studied compounds was relatively low, the most efficient inhibitors were 5-tert-butyl-6-chloropyrazine-2-carboxylic acid (4-fluorophenyl)-amide (2d), 5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (3-chlorophenyl)-amide (2h, both IC50 = 103.0 µmol dm-3), and especially 5-tert-butylpyrazine-2-carboxylic acid (3-chlorophenyl)-amide (2g, IC50 = 47.0 µmol dm-3). Their log P values calculated ranged between 3.28 and 4.18.
Some of the compounds under study reduced the chlorophyll content in Chlorella vulgaris Beij. IC50 value could be determined only for 6-chloropyrazine-2-carboxylic acid (4-fluorophenyl)amide (2b, IC50 = 32.3 µmol dm-3), i.e. 4 times lower activity value, than one's for the standard (DCMU) determined was. Most of the compounds studied were inactive due to their poor aqueous solubility.
No conclusive finding for the structure-activity relationships in the mentioned series was obtained. Only low importance of increased hydrophobic properties for photosynthesis-inhibiting evaluation was determined in compounds studied was achieved. In contrast with our anticipation, the biological activity in the series studied the compounds with the grouping 2-Cl-5-OH- on benzene ring was not high. Antimycobacterial evaluation of newly prepared pyrazine-2-carboxylic acid derivatives will be an additional area of our interest.

Scheme 1: Synthesis of substituted pyrazinecarboxylic acid 1a-d.
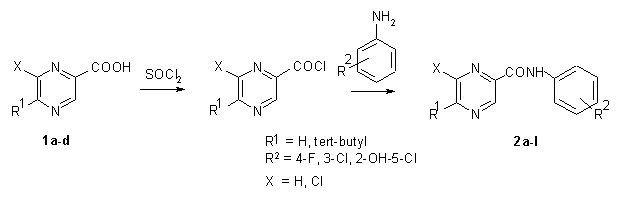
Scheme 2: Synthesis of some substituted pyrazine-2-carboxamides 2a-l.
All organic solvents used for the synthesis were of analytical grade. The solvents were dried and freshly distilled under argon atmosphere. TLC was performed on Silufol UV 254 plates (Kavalier, Votice, Czech Republic) in the following solvent system: acetone/toluene (1:1). The spots were detected in UV (254 nm). Melting points were determined on Boetius PHMK 05 (VEB Kombinat Nagema, Radebeul, Germany). Elemental analyses were obtained using an EA 1110 CE instrument (Fisons Instruments S.p.A., Milan). Infrared spectra were recorded in KBr pellets on an IR-spectrometer Nicolet Impact 400. 1H and 13C NMR Spectra were recorded on Varian Mercury – Vx BB 300 (299.95 MHz for 1H and 75.43 MHz for 13C), Varian (Palo Alto CA, USA). Chemical shifts are given relative to internal Si(CH3)4. Log P values were computed using the program CS ChemOffice Ultra ver. 7.0 (CambridgeSoft, Cambridge MA, USA).
A mixture of acid, i.e. pyrazine-2-carboxylic [18,19], 6-chloropyrazine-2-carboxylic [20], 5-tert-butylpyrazine-2-carboxylic [12] or 5-tert-butyl-6-chloropyrazine-2-carboxylic [12] acids, respectively, (50.0 mmol) and thionyl chloride (5.5 mL, 75.0 mmol) in 20 mL of dry toluene was refluxed for about 1 h. Excess of thionyl chloride was removed by repeated evaporation with dry toluene in vacuo. The crude acyl chloride dissolved in 50 mL of dry acetone was added drop wise to a stirred solution of the corresponding substituted amine (50.0 mmol) in 50 mL of dry pyridine keeping at the room temperature. After the addition was complete, stirring continued for another 30 min. The reaction mixture was then poured into 100 mL of cold water and the crude amide was collected and recrystallized from aqueous ethanol.
Pyrazine-2-carboxylic acid (4-fluorophenyl)amide (2a). Yield: 82.6%, m.p. 154-155 °C. For C11H8FN3O (217.2) calculated: 60.83 % C, 3.71% H, 19.35% N; found: 60.75% C, 3.74% H, 19.40% N. IR spectrum (KBr), cm-1: 3428 (N-H), 1680 (C=O). 1H NMR (300 MHz, DMSO) δ 10.84 (1H, bs, NH), 9.29 (1H, d, J=1.7 Hz, H3), 8.92 (1H, d, J=2.5 Hz, H6), 8.80 (1H, dd, J=2.5 Hz, J=1.4 Hz, H5), 7.98-7.87 (2H, m, H2', H6'), and 7.26-7.15 (2H, m, H3', H5'). 13C NMR (75 MHz, DMSO) δ 161.9, 158.8 (d, J=240.8 Hz), 148.0, 145.2, 144.3, 143.4, 134.8 (d, J=2.6 Hz), 122.7 (d, J=7.8 Hz), and 115.5 (d, J=22.0 Hz).
6-Chloropyrazine-2-carboxylic acid (4-fluorophenyl)amide (2b). Yield: 93.2%, m.p. 131-132 °C. For C11H7ClFN3O (251.7) calculated: 52.50 % C, 2.80% H, 16.70% N; found: 52.38% C, 2.91% H, 16.63% N. IR spectrum (KBr), cm-1: 3439 (N-H), 1685 (C=O). 1H NMR (300 MHz, DMSO) δ 10.74 (1H, bs, NH), 9.22 (1H, d, J=0.6 Hz, H3), 9.05 (1H, s, H5), 7.93-7.83 (2H, m, H2', H6'), and 7.27-7.17 (2H, m, H3', H5'). 13C NMR (75 MHz, DMSO) δ 160.8, 159.0 (d, J=241.0 Hz), 147.7, 147.1, 145.3, 142.6, 134.5 (d, J=2.6 Hz), 123.0 (d, J=8.1 Hz), and 115.5 (d, J=22.3 Hz).
5-tert-Butylpyrazine-2-carboxylic acid (4-fluorophenyl)amide (2c). Yield: 90.8%, m.p. 178 °C. For C11H16FN3O (273.3) calculated: 65.92 % C, 5.90% H, 15.37% N; found: 66.03% C, 5.81% H, 15.30% N. IR spectrum (KBr), cm-1: 3436 (N-H), 1678 (C=O). 1H NMR (300 MHz, DMSO) δ 10.45 (1H, bs, NH), 9.19 (1H, d, J=1.5 Hz, H3), 8.84 (1H, d, J=1.5 Hz, H6), 7.96-7.86 (2H, m, H2', H6'), 7.25-7.15 (2H, m, H3', H5'), and 1.39 (9H, s, CH3). 13C NMR (75 MHz, DMSO) δ 166.9, 162.0, 158.7 (d, J=240.8 Hz), 142.6, 142.6, 139.9, 134.9 (d, J=2.6 Hz), 122.6 (d, J=8.0 Hz), 115.5 (d, J=22.3 Hz), 37.0, and 29.6.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (4-fluorophenyl)amide (2d). Yield: 94.3%, m.p. 171-172 °C. For C15H15ClFN3O (307.8) calculated: 58.54 % C, 4.91% H, 13.65% N; found: 58.49% C, 5.06% H, 13.58% N IR spectrum (KBr), cm-1: 3439 (N-H), 1681 (C=O). 1H NMR (300 MHz, DMSO) δ 10.62 (1H, bs, NH), 9.12 (1H, s, H3), 7.91-7.81 (2H, m, H2', H6'), 7.26-7.16 (2H, m, H3', H5'), and 1.50 (9H, s, CH3). 13C NMR (75 MHz, DMSO) δ 163.0, 160.9, 158.9 (d, J=241.4 Hz), 145.4, 142.8, 140.6, 134.6 (d, J=2.6 Hz), 122.9 (d, J=8.0 Hz), 115.5 (d, J=22.3 Hz), 38.7, and 28.2.
Pyrazine-2-carboxylic acid (3-chlorophenyl)amide (2e). Yield: 72.8%, m.p. 139-140 °C. For C11H8ClN3O (233.7) calculated: 56.54 % C, 3.45% H, 17.98% N; found: 56.53% C, 3.51% H, 18.03% N. IR spectrum (KBr), cm-1: 3435 (N-H), 1673 (C=O). 1H NMR (300 MHz, CDCl3) δ 9.68 (bs, 1H, NH), 9.50 (s, 1H, H3), 8.83 (d, 1H, J=2.19 Hz, H6), 8.62-8.57 (m, 1H, H5), 7.92-7.86 (m, 1H, H2'), 7.65-7.56 (m, 1H, H6'), 7.31 (t, 1H, J=1.97 Hz, H5'), and 7.18-7.11 (m, 1H, H4'). 13C NMR (75 MHz, CDCl3) δ 160.7, 147.7, 144.7, 144.0, 142.4, 138.3, 134.8, 130.2, 124.9, 119.9, and 117.7.
6-Chloropyrazine-2-carboxylic acid (3-chlorophenyl)amide (2f). Yield: 90.7%, m.p. 107-108 °C. For C11H7Cl2N3O (268.1) calculated: 49.28% C, 2.63% H, 15.67% N; found: 49.33% C, 2.61% H, 15.63% N. IR spectrum (KBr), cm-1: 3435 (N-H), 1676 (C=O). 1H NMR (300 MHz, CDCl3) δ 9.44-9.35 (m, 2H, NH, H3), 8.82 (s, 1H, H5), 7.88 (t, 1H, J=1.93 Hz, H2'), 7.60 (ddd, 1H, J=7.97 Hz, J=1.93 Hz, J=0.83 Hz, H6'), 7.32 (t, 1H, J=7.96 Hz, H5'), and 7.17 (ddd, 1H, J=7.97 Hz, J=1.92 Hz, J=0.82 Hz, H4'). 13C NMR (75 MHz, CDCl3) δ 159.4, 147.8, 147.5, 143.6, 142.2, 137.9, 134.9, 130.2, 125.2, 120.1, and 118.0.
5-tert-Butylpyrazine-2-carboxylic acid (3-chlorophenyl)amide (2g). Yield: 82.5%, m.p. 117-118 °C. For C15H16ClN3O (289.8) calculated: 62.18% C, 5.57% H, 14,50% N; found: 62.15% C, 5.51% H, 14.59% N. IR spectrum (KBr), cm-1: 3440 (N-H), 1685 (C=O).1H NMR (300 MHz, CDCl3) δ 9.67 (bs, 1H, NH), 9.38 (d, 1H, J=1.37 Hz, H3), 8.62 (d, 1H, J=1.37 Hz, H6), 7.89 (t, 1H, J=2.07 Hz, H2'), 7.59 (ddd, 1H, J=7.96 Hz, J=2.07 Hz, J=1.10 Hz, H4'), 7.30 (t, 1H, J=7.96 Hz, H5'), 7.13 (ddd, 1H, J=7.96 Hz, J=2.07 Hz, J=1.10 Hz, H6'), and 1.45 (s, 9H, CH3). 13C NMR (75 MHz, CDCl3) δ 168.0, 161.1, 143.0, 141.0, 139.0, 138.5, 134.8, 130.1, 124.6, 119.8, 117.6, 37.1, and 29.7.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (3-chlorophenyl)amide (2h). Yield: 97.1%, m.p. 86-87 °C. For C15H15Cl2N3O (324.2) calculated: 55.57% C, 4.66% H, 12.96% N; found: 55.45% C, 4.63% H, 13.08% N. IR spectrum (KBr), cm-1: 3432 (N-H), 1678 (C=O).1H NMR (300 MHz, CDCl3) δ 9.39 (bs, 1H, NH), 9.26 (s, 1H, H3), 7.88 (t, 1H, J=2.07 Hz, H2'), 7.60 (ddd, 1H, J=7.97 Hz, J=2.07 Hz, J=1.10 Hz, H6'), 7.31 (t, 1H, J=7.97 Hz, H5'), 7.15 (ddd, 1H, J=7.97 Hz, J=2.07 Hz, J=1.10 Hz, H4'), and 1.55 (s, 9H, CH3). 13C NMR (75 MHz, CDCl3) δ 164.9, 159.9, 145.8, 140.7, 140.3, 138.2, 134.8, 130.1, 125.0, 120.0, 117.9, 39.0, and 28.20.
Pyrazine-2-carboxylic acid (2-chloro-5-hydroxyphenyl)amide (2i). Yield: 81.6%, m.p. 223-224 °C. For C11H8ClN3O2 (249.5) calculated: 52.92 % C, 3.23% H, 16.83% N; found: 53.03% C, 3.21% H, 16.73% N. IR spectrum (KBr), cm-1: 3432 (N-H), 1670 (C=O). 1H NMR (300 MHz, DMSO) δ 10.27 (1H, bs, NH), 9.93 (1H, bs, OH), 9.32 (1H, d, J=1.4 Hz, H3), 8.97 (1H, d, J=2.5 Hz, H6), 8.83-8.81 (1H, m, H5), 7.87 (1H, d, J=2.7 Hz, H6'), 7.32 (1H, d, J=8.8 Hz, H3'), and 6.61 (1H, dd, J=8.8 Hz, J=2.7 Hz, H4'). 13C NMR (75 MHz, DMSO) δ 160.9, 157.1, 148.7, 144.0, 143.9, 143.6, 134.5, 130.0, 113.5, 113.2, and 109.3.
6-Chloropyrazine-2-carboxylic acid (2-chloro-5-hydroxyphenyl)amide (2j). Yield: 87.9%, m.p. 255 °C. For C11H7Cl2N3O2 (284.1) calculated: 46.50% C, 2.48% H, 14.79% N; found: 46.51% C, 2.41% H, 14.83% N. IR spectrum (KBr), cm-1: 3438 (N-H), 1683 (C=O). 1H NMR (300 MHz, DMSO) δ 10.10 (1H, bs, NH), 9.93 (1H, bs, OH), 9.26 (1H, s, H3), 9.11 (1H, s, H5), 7.71 (1H, d, J=2.8 Hz, H6'), 7.33 (1H, d, J=8.7 Hz, H3'), and 6.64 (1H, dd, J=8.7 Hz, J=2.8 Hz, H4'). 13C NMR (75 MHz, DMSO) δ 159.9, 157.1, 148.3, 147.0, 143.9, 142.2, 134.4, 130.0, 114.4, 113.7, and 110.2.
5-tert-Butylpyrazine-2-carboxylic acid (2-chloro-5-hydroxyphenyl)amide (2k). Yield: 76.2%, m.p. 239-240 °C. For C15H16ClN3O2 (305.8) calculated: 58.92% C, 5.27% H, 13.74% N; found: 58.83% C, 5.31% H, 13.82% N. IR spectrum (KBr), cm-1: 3435 (N-H), 1677 (C=O). 1H NMR (300 MHz, DMSO) δ 10.24 (1H, bs, NH), 9.93 (1H, bs, OH), 9.23 (1H, d, J=1.6 Hz, H3), 8.93 (1H, d, J=1.6 Hz, H6), 7.92 (1H, d, J=2.9 Hz, H6'), 7.32 (1H, d, J=8.8 Hz, H3'), 6.60 (1H, dd, J=8.8 Hz, J=2.9 Hz, H4'), and 1.39 (9H, s, CH3). 13C NMR (75 MHz, DMSO) δ 167.8, 160.9, 157.2, 142.3, 141.2, 140.5, 134.6, 129.9, 113.1, 113.0, 108.9, 37.1, and 29.6.
5-tert-Butyl-6-chloropyrazine-2-carboxylic acid (2-chloro-5-hydroxyphenyl)amide (2l). Yield: 94.4%, m.p. 253 °C. For C15H15Cl2N3O2 (340.2) calculated: 52.96% C, 4.44% H, 12.35% N; found: 52.93% C, 4.48% H, 12.39% N. IR spectrum (KBr), cm-1: 3440 (N-H), 1675 (C=O). 1H NMR (300 MHz, DMSO) δ 10.03 (1H, bs, NH), 9.93 (1H, bs, OH), 9.16 (1H, s, H3), 7.76 (1H, d, J=2.8 Hz, H6'), 7.33 (1H, d, J=8.7 Hz, H3'), 6.63 (1H, dd, J=8.7 Hz, J=2.8 Hz, H4'), and 1.50 (9H, s, CH3). 13C NMR (75 MHz, DMSO) δ 163.8, 159.9, 157.1, 145.3, 141.4, 140.3, 134.4, 130.0, 114.0, 113.5, 109.8, 38.8, and 28.2.
The broth microdilution test [24,25] was used for the assessment of in vitro antifungal activity of the synthesized compounds and ketoconazole (standard) against Candida albicans ATCC 44859, Candida tropicalis 156, Candida krusei E28, Candida glabrata 20/I, Trichosporon beigelii 1188, Trichophyton mentagrophytes 445, Aspergillus fumigatus 231, and Absidia corymbifera 272. The procedure was performed with twofold dilutions of the compounds in RPMI 1640 buffered to pH 7.0 with 0.165 mol of 3-morpholino-propane-1-sulfonic acid. The final concentrations of the compounds ranged from 1000 to 0.975 µmol l-1. Drug–free controls were included. The MICs were determined after 24 h and 48 h of static incubation at 35 °C. With Trichophyton mentagrophytes, the final MICs were read after 72 h and 120 h of incubation. For the results see Table 1.
Table 1. In vitro antifungal susceptibility testing of amides 2a-l in comparison with ketoconazole (KET).
| Comp. | MIC (µmol l-1) | |||||||
| TM | CA | CT | CK | CG | TB | AF | AC | |
| 72h 120h |
24h 48h |
24h 48h |
24h 48h |
24h 48h |
24h 48h |
24h 48h |
24h 48h |
|
| 2a | 125 125 |
62.5 125 |
500 |
250 >500 |
>500 >500 |
125 250 |
125 250 |
250 250 |
| 2b | 250 250 |
62.5 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
250 250 |
125 125 |
250 250 |
| 2c | 125 125 |
>125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
125 125 |
| 2d | >125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
>125 >125 |
62.5 125 |
>125 >125 |
| 2e | 500 >500 |
250 500 |
500 >500 |
500 >500 |
>500 >500 |
500 >500 |
250 >500 |
>500 >500 |
| 2f | 125 125 |
62.5 62.5 |
500 >500 |
250 >500 |
>500 >500 |
125 250 |
125 250 |
250 250 |
| 2g | >250 >250 |
250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
| 2h | >250 >250 |
250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
| 2i | 250 250 |
500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
| 2j | 125 125 |
125 250 |
>500 >500 |
500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
>500 >500 |
| 2k | 125 125 |
62.5 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
250 >250 |
| 2l | >250 >250 |
125 125 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
>250 >250 |
250 125 |
| KET | 0.98 1.95 |
<0.24 <0.24 |
1.95 3.91 |
0.98 1.95 |
0.49 1.95 |
<0.24 <0.24 |
7.81 7.81 |
31.25 31.25 |
Strains tested: | |
| 1. CA-Candida albicans ATCC 44859 | 5. TB-Trichosporon beigelii 1188 |
| 2. CT-Candida tropicalis 156 | 6. TM-Trichophyton mentagrophytes 445 |
| 3. CK-Candida krusei E28 | 7. AF-Aspergillus fumigatus 231 |
| 4. CG-Candida glabrata 20/I | 8. AC-Absidia corymbifera 272 |
The oxygen evolution rate in spinach chloroplasts was investigated spectrophotometrically (Specord UV VIS, Zeiss, Jena) in the presence of an electron acceptor 2,6-dichlorophenol-indophenol, by method described in Ref. [26]. The compounds were dissolved in dimethyl sulfoxide (DMSO) because of their low water solubility. The used DMSO volume fractions (up to 5 vol. %) did not affect the oxygen evolution. The inhibitory efficiency of the studied compounds has been expressed by IC50 values, i.e. by molar concentration of the compounds causing 50 % decrease in the oxygen evolution relative to the untreated control. IC50 value for the standard, a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (DIURON) was measured about 1.9 µmol dm-3. For the results see Table 2.
Table 2. Structure, inhibition of oxygen evolution rate in spinach chloroplasts (IC50, µmol dm-3) and lipophilicity (log P) of compounds 2a-l in comparison with standard (DCMU).
| Comp. | X | R1 | R2 | IC50 (µmol dm-3) |
log P |
| 2a | H | H | 4-F | 480.0 | 0.72 |
| 2b | Cl | H | 4-F | 384.0 | 1.65 |
| 2c | H | (CH3)3C | 4-F | 524.0 | 2.88 |
| 2d | Cl | (CH3)3C | 4-F | 103.0 | 3.78 |
| 2e | H | H | 3-Cl | 290.0 | 1.20 |
| 2f | Cl | H | 3-Cl | 262.0 | 2.05 |
| 2g | H | (CH3)3C | 3-Cl | 47.0 | 3.28 |
| 2h | Cl | (CH3)3C | 3-Cl | 103.0 | 4.18 |
| 2i | H | H | 2-Cl-5-OH | 722.0 | 0.76 |
| 2j | Cl | H | 2-Cl-5-OH | 624.0 | 1.66 |
| 2k | H | (CH3)3C | 2-Cl-5-OH | a | 2.89 |
| 2l | Cl | (CH3)3C | 2-Cl-5-OH | 652.0 | 3.79 |
| DCMU | - | - | - | 1.9 | 2.32 |
a not tested due to their low solubility in DMSO
The green algae Chlorella vulgaris Beij. were cultivated statically at room temperature according to Kralova et al. [27] (photoperiod 16 h light/8 h dark; illumination 4000 lx; pH = 7.2). The effect of compounds 2a-l on algal chlorophyll (Chl) content was determined after 7-day cultivation in the presence of the compounds tested, expressing the response as percentage of the corresponding values obtained for control. The Chl content in the algal suspension was determined spectrophotometrically (Specord UV VIS, Zeiss Jena, Germany) after extraction into methanol according to Wellburn [28]. The Chl content in the suspensions at the beginning of cultivation was 0.1 mg dm-3. Because of their low water solubility, the tested compounds were dissolved in dimethyl sulfoxide (DMSO). DMSO concentration in the algal suspensions did not exceed 0.25 v/v % and the control samples contained the same DMSO amount as the suspensions treated with the tested compounds. IC50 value (the concentration of the inhibitor causing a 50% decrease in the content of Chl as compared with the control sample) for the standard, a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (DIURON) was measured about 7.3 µmol dm-3. Some partial precipitation of samples in solution tested was occurred in the series of amides 2a-l, therefore only at the compound 2b the IC50 value (32.3 µmol dm-3) was successfully made out.
Acknowledgements. This study was supported by IGA Ministry of Health of the Czech Republic No. 1A/8238-3 and by the Slovak Scientific Grant Agency VEGA (No. 1/0089/03). We also thank Mrs. I. Vencovska, and Mr. T. Vojtisek from the Faculty of Pharmacy in Hradec Kralove, Charles University in Prague, Czech Republic, for their technical assistance.
1. Fostel, J.M.; Lartey P.A. Drug Discovery Today 2000, 5, 25.
2. Fromtling, J.M. Drug News & Perspectives 1997, 10, 557.
3. Dolezal, M. Cesk. Slov. Farm. 2002, 51, 226.
4. Tsuda, T.; Kobayashi, K.; Ichimoto, I. Nippon Noyaku Gakkaishi (日本農薬学会) 1997, 22, 218; ref. Chem. Abstr. 1997, 127, 220 630m.
5. Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Chem. Pap. 1999, 53, 328.
6. Miletin, M.; Hartl, J.; Machacek, M. Collect. Czech. Chem. Commun. 1997, 62, 672.
7. Kralova, K.; Loos, D.; Miletin, M.; Klimesova, V. Folia Pharm. Univ. Carol. 1998, 23 Suppl., 77.
8. Miletin, M.; Dolezal, M.; Hartl, J.; Kralova, K.; Machacek M. 3th Int. Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), September 1-30, 1999 (http://www.unibas.ch/mdpi/ecsoc-3/c0005/c0005.htm).
9. Kralova, K.; Loos, D.; Cizmarik, J. Collect. Czech. Chem. Commun. 1994, 59, 2293.
10. Kralova, K.; Loos, D.; Cizmarik, J. Photosynthetica 1994, 30, 155.
11. Kralova, K.; Bujdakova, H.; Cizmarik, J. Pharmazie 1995, 50, 440.
12. Dolezal, M.; Hartl, J.; Miletin, M.; Machacek, M.; Kralova K. Chem. Pap. 1999, 53, 126.
13. Dolezal, M.; Vicik, R.; Miletin, M.; Kralova, K. Chem. Pap. 2000, 54, 245.
14. Dolezal, M.; Miletin, M.; Hartl, J.; Kralova, K.; Kunes, J. 4th Int. Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), September 1-30, 2000 (http://www.unibas.ch/mdpi/ecsoc-4/c0028/c0028.htm).
15. Dolezal, M.; Hartl, J.; Miletin, M. Folia Pharm. Univ. Carol. 2000, 25, 15 (http://www.faf.cuni.cz/folia/25/2502dolezal.htm).
16. Dolezal, M.; Miletin, M.; Hejsky, R.; Kralova, K.; Kunes, J. 5th Int. Electronic Conference on Synthetic Organic Chemistry (ECSOC-5), September 1-30, 2001 (http://www.mdpi.net/ecsoc-5/c0009/c0009.htm).
17. Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Molecules 2002, 7, 363 (http://www.mdpi.net/molecules/papers/70300363.pdf).
18. Kushner, S.; Dalalian, H.; Sanjurjo, J.L.; Bach, F.L.; Safir, S.R.; Smith, V.K.; Williams, J.H. J. Amer. Chem. Soc. 1952, 74, 3617.
19. Foks, H.; Salewicz, J. Acta Polon. Pharm. 1964, 21, 429.
20. Abe, Y.; Shigeta, Y.; Uchimaru, F.; Okada, S.; Ozasayama, E. Japan. Pat. 1969, 69 12,898; ref. Chem. Abstr. 1969, 71, 112979y.
21. Dolezal, M.; Kutilova, H.; Kralova, K.; Kunes, J. 6th Int. Electronic Conference on Synthetic Organic Chemistry (ECSOC-6), September 1-30, 2002 (http://www.mdpi.net/ec/papers/ecsoc-6/213/213.htm).
22. Jegerschöld, C.; Styring, S. FEBS Lett. 1991, 280, 87.
23. Dolezal, M.; Kralova, K.; Sersen, F.; Miletin, M. Folia Pharm. Univ. Carol. 2001, 26, 13 (http://www.faf.cuni.cz/folia/26/2602dolezal.htm).
24. National Committee for Clinical Laboratory Standards: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Proposed Standard M 27-P, National Committee for Clinical Laboratory Standards, Villanova, Pa, 1992.
25. Sheehan, D.J.; Espinel-Ingroff, A.; Steele, M.; Webb, C.D. Clin. Infect. Dis. 1993, 17 494.
26. Kralova, K.; Sersen, F.; Sidoova, E. Chem. Pap. 1992, 46, 348.
27. Kralova, K.; Sersen, F.; Melnik, M. J. Trace Microprobe Techn. 1998, 16, 491.
28. Wellburn, A.R. J. Plant. Physiol. 1994, 144, 307.