8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. 1-30 November 2004. http://www.lugo.usc.es/~qoseijas/ECSOC-8/
[C009]
Efficient synthesis of 2-substituted cyclobutanones as markers for food irradiation
Guido Verniest, Stefaan Boterberg, Jeroen Colpaert, Tinneke Van Thienen, Christian V. Stevens, Norbert De Kimpe*
Department of Organic Chemistry, Faculty of Agricultural and Applied Biological Sciences, Ghent University, Coupure Links 653, B-9000 Gent, Belgium
Fax: +32 (0)9 264 62 43
E-mail: [email protected]
Abstract: A short and efficient synthesis is described towards 2-dodecyl-, 2-tetradecyl, 2-(5-tetradecenyl)- and 2-(5,8-tetradecadienyl)cyclobutanones, which are specific markers of gamma-irradiated foodstuffs, e.g. chicken meat and liquid whole egg.
Ionizing radiation, e.g. γ-irradiation, for the preservation of foods is not generally accepted and allowed. The development of tests for the detection of irradiated foods is of importance in this matter.1 Together with a good management at the irradiation facility; such tests would facilitate international trade and increase consumer confidence in the existing control procedures. At present, reliable detection methods for irradiated foods include electron spin resonance to monitor long-lived radicals, thermoluminiscence, detection of o-tyrosine formed in meat, and the detection of volatile compounds from irradiated fats.2 The detection of 2-substituted cyclobutanones as markers for γ-irradiated foods proved to be very successful for chicken,1,2c,2f,3,4 peanuts,5 papaya,2f,6 liquid whole egg,2e,3 pork, lamb, beef3 and fish.7 These 2-substituted cyclobutanones are formed from the γ-irradiation induced cyclization of triglycerides 1 or fatty acids 2, and were shown to contain the same number of carbon atoms as the parent fatty acids (Scheme 1).

Scheme 1
As palmitic acid, stearic acid, oleic acid and linoleic acid are the four major fatty acids found in most foods, the presence of 2-dodecylcyclobutanone 3a, 2-tetradecylcyclobutanone 3b, 2-(tetradec-5-enyl)cyclobutanone 4 and 2-(tetradeca-5,8-dienyl)cyclobutanone 5 was proven in foods following irradiation. Methods using GC-MS have been developed for the detection of such 2-substituted cyclobutanones 3-5 in foods (containing minimum 1% of fat) irradiated with 500Gy or even less.4,8 One of these cyclobutanone detection methodologies has been selected by the European Committee for Standardization to prove food irradiation.9 The success of the detection of 2-substituted cyclobutanones is dependant upon the ready availability of the standard compounds by chemical synthesis. In recent years, efforts have been performed to make such cyclobutanones 3-5 accessible, but most of the synthetic pathways are too long, using reagents which are difficult to handle e.g. sensitive cyclopropanes and the pyrophoric tert-butyllithium.9,10
This paper describes the alkylation and alkenylation of N-(cyclobutylidene)isopropylamine as a high yielding, easy to perform and efficient route towards cyclobutanones 3-5. Only very recently, a synthesis of 2-alkylated cyclobutanones was published via the alkylation of cyclobutanone hydrazone.11 However, this simultaneous12 research did not offer an efficient synthesis of the most important and specific alkenylated cyclobutanone 4, because a Wittig-Horner reaction was used as a key step towards 1-bromo-5-tetradecene resulting in a mixture of E- and Z-isomers.11 Also, no synthesis of alkadienylcyclobutanone 5 was reported in this article.11

Scheme 2
Commercially available cyclobutanone 6 was converted into N-(cyclobutylidene)isopropylamine 713 by reaction with isopropylamine (3.5 equiv.) in diethyl ether in the presence of stoichiometric amounts of titanium(IV) chloride which acts as an activator and chemical dehydrating agent. Without purification, the cyclobutanone imine 7 was deprotonated with LDA in THF at -78°C and the resulting 1-azaallylic anion intermediate was reacted with various alkylbromides to afford the corresponding N-(2-alkyl-1-cyclobutylidene)isopropylamines 8. The latter crude imines 8 were hydrolyzed with aqueous oxalic acid in a two-phase system under reflux resulting in 2-substituted cyclobutanones 3 in 71-80% yield. Besides 2-dodecyl- and 2-tetradecylcyclobutanone, which are ubiquitous in irradiated lipid containing foods, also the less occurring hexyl-, octyl- and decylcyclobutanone were synthesized using this procedure. Further purification was performed by flash chromatography giving rise to the pure standards, useful for the detection of markers for irradiated foodstuffs (Scheme 2).
Using the same methodology, a one-pot procedure was evaluated to access cyclobutanones 3 by imination of the starting cyclobutanone 6 and direct alkylation of the formed imine after filtration. Hydrolysis of the alkylated imines 8 was performed in situ by adding aqueous oxalic acid. The yields of the cyclobutanones synthesized by this procedure were practically the same as compared with the multi-pot synthesis. However, the ease of performing this three-step synthesis in one pot, makes this procedure very suitable for the synthesis of 2-alkylated cyclobutanones 3. Independently and prior12 to the recently reported synthesis of 2-alkylcyclobutanones by Miesch et al.,11 we also evaluated the use of N-cyclobutylidene-(N’,N’-dimethylamino)amine 914 as a starting material for the alkylation reaction. This procedure did not prove to give superior yields as compared with the one-pot synthesis (Scheme 3).12
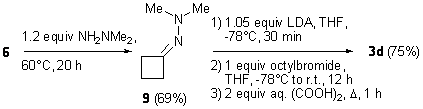
Scheme 3
To synthesize the more specific unsaturated cyclobutanone derivatives 4 and 5, the corresponding olefinic halides were synthesized in order to use these substrates in the alkylation step. Of prime importance is the fact that the final alkenyl- and alkadienylcyclobutanones are obtained stereochemically pure.

Scheme 4
Commercially available 5-hexyn-1-ol 10 was deprotonated by reaction with 2.2 equivalents of BuLi and was subsequently reacted with 1-iodooctane to yield tetradec-5-ynol 11. Partial reduction of the triple bond using the Lindlar catalyst under H2-atmosphere, afforded tetradecenol 1215 in high yield. Functional group transformation of the hydroxy moiety of 12 towards 1-bromotetradecene 13 was accomplished using PBr3 in dichloromethane (Scheme 4). For the synthesis of 1-bromo-5,8-tetradecadiene 17, a copper(I) catalyzed cross-coupling of 5-hexyn-1-ol 10 and 1-bromo-2-octyn 14 was used,16 followed by partial reduction of 15. To transform the obtained 5,8-tetradecadienol into the 1-bromodiene 17, 1617 was reacted with triphenylphosphine and tetrabromomethane in dichloromethane at 0°C and afforded 1-bromodiene 17 in high yield (Scheme 5).

Scheme 5
Finally, the obtained bromoalkenes 13 and 17 were used for the alkylation of cyclobutanone. The same procedure was followed as for the synthesis of the saturated derivatives 3. In this way, a new efficient route is established towards 2-alkenylcyclobutanones 4 and 5, which can be used as markers for irradiated foodstuffs.
SELECTED EXPERIMENTAL PROCEDURES
5,8-Tetradecadiyn-1-ol (15). To a suspension of 1.46 g (7.66 mmol, 2 equiv.) CuI, 1.15 g (7.66 mmol, 2 equiv.) NaI, 0.80 g (5.74 mmol, 1.5 equiv.) K2CO3 in 10 mL of DMF, 1-bromo-2-octyn 14 (0.80 g, 4.21 mmol, 1.1 equiv.) and 5-hexyn-1-ol 10 (0.38 g, 3.83 mmol, 1 equiv.) were added under N2-atmosphere at room temperature.16
After stirring for 15 h, the suspension was filtered over Celite® and the filtrate was poured into 20 mL of saturated aqueous NH4Cl and extracted with diethyl ether (3x 30 mL). Drying and evaporation of the solvent resulted in a pale oil which was chromatographed on silicagel (diethyl ether/hexane: 25/75, Rf = 0.11), yield: 87%. 1H NMR (CDCl3, 300 MHz) δ 0.90 (t, J= 6.8Hz, 3H), 1.25-1.40 (m, 4H), 1.43-1.79 (m, 6H), 2.05 (s(br), 1H), 2.15 (tt, J= 7.2Hz; 2.4Hz, 2H), 2.21 (tt, J= 6.7Hz; 2.4Hz, 2H), 3.12 (quintet, J= 2.4Hz, 2H), 3.66 (t, J= 6.5Hz, 2H). 13C NMR (CDCl3, 75 MHz) δ 9.75, 14.04, 18.56, 18.74, 22.27, 25.02, 28.51, 31.14, 31.86, 62.43, 74.41, 75.05, 80.07, 80.67. IR (KBr) νmax 3369 cm−1. MS m/z (%) 206 (M+, 2), 205 (5), 191 (2), 149 (37), 110 (74), 91 (100), 79 (54). Anal. Calcd. for C14H22O: C, 81.50; H, 10.75. Found: C, 81.71; H, 10.94.
(5Z,8Z)-1-Bromo-5,8-tetradecadiene (17). To a solution of 0.25 g (1.20 mmol) tetradecadienol 16 in 5 mL of dry CH2Cl2 at 0°C was added 0.47 g (1.79 mmol, 1.5 equiv.) triphenylphosphine and 0.59 g (1.79 mmol, 1.5 equiv.) dried CBr4. The mixture was allowed to reach room temperature and was stirred for 8 h at this temperature. The solids were filtered and the filtrate was evaporated in vacuo. Purification of the obtained bromide 17 was accomplished by flash chromatography (diethyl ether/hexane: 2/8, Rf = 0.75), yield: 90%. 1H NMR (CDCl3, 300 MHz) δ 0.89 (t, J= 6.7Hz, 3H), 1.29-1.41 (m, 6H), 1.52 (quint, J= 7.3Hz, 2H), 1.88 (quint, J= 7.3Hz, 2H), 2.02-2.13 (m, 4H), 2.77 (t, J= 5.6Hz, 2H), 3.41 (t, J= 6.7Hz, 2H), 5.27-5.44 (m, 4H). 13C NMR (CDCl3, 75 MHz) δ 14.19, 22.68, 25.72, 26.39, 27.32, 28.19, 29.43, 31.63, 32.41, 33.86, 127.73, 128.93, 129.18, 130.49. IR (KBr) νmax 1648, 1457, 670 cm−1. MS m/z (%) 272/4 (M+, 13), 188/90 (14), 95 (57), 81 (86), 67 (100). Anal. Calcd. for. C14H25Br: C, 61.54; H, 9.22. Found: C, 61.78; H, 9.40.
Acknowledgment
The authors are indebted to the IWT (Flemish Institute for the Promotion of Scientific-Technological Research in Industry), the FWO-Flanders and Ghent University (GOA) for financial support.
References
(1) Stevenson, M. H.; Crone, A. V. J.; Hamilton, J. T. G. Nature 1990, 334, 202.
(2) (a) Stevenson, M. H.; Gray, R. J. Sci. Food Agric. 1989, 48, 261. (b) Lea, J. S.; Dodd, N. J. F. Int. J. Food Sci. Technol. 1988, 23, 625. (c) Boyd, D. R.; Crone, A. V. J.; Hamilton, J. T. G.; Hand, M. V.; Stevenson, M. H.; Stevenson, P. J.J. Agric. Food Chem. 1991, 39, 789. (d) Stevenson, M. H. Trends in Food Science & Technol. 1992, 31, 257. (e) Crone, A. V. J.; Hand, M. V.; Hamilton, J. T. G.; Sharma, N. D.; Boyd, D. R.; Stevenson, M. H. J. Sci. Food Agric. 1993, 62, 361. (f) Hamilton, L.; Stevenson, M. H.; Boyd, D. R.; Branningen, I. N.; Treacy, A. B.; Hamilton, J. T. G.; McRoberts, W. C.; Elliott, C. T. J. Chem. Soc., Perkin Trans. 1 1996, 139.
(3) Stevenson, M. H. Food Technol. 1994, 141.
(4) Ndiaye, B.; Horvatovich, P.; Miesch, M.; Hasselmann, C.; Marchioni, E. J. Chromatogr. A 1999, 858, 109.
(5) Lee, H.-J.; Lee, M.-Y.; Kim, K.-S. J. Food Sci. Nutr. 1999, 4, 270.
(6) Stewart, E. M.; Moore, S.; Graham, W. D.; McRoberts, W. C.; Hamilton, J. T. G. J. Sci. Food Agr. 2000, 80, 121.
(7) Tewfik, I. H.; Ismael, H. M.; Sumar, S. Int. J. Food Sci. Nutr. 1999, 50, 51.
(8) (a) Horvatovich, P.; Miesch, M.; Hasselmann, C.; Marchioni, E. J. Chromatogr. A 2000, 897, 259. (b) Horvatovich, P.; Miesch, M.; Hasselmann, C.; Marchioni, E. J. Chromatogr. A 2000, 968, 251.
(9) Miesch, M.; Ndiaye, B.; Hasselmann, C.; Marchioni, E. Radiat. Phys. Chem. 1999, 55, 337.
(10) (a) Miller, S. A.; Gadwood, R. C. Org. Synth. Coll. Vol. 1993, 8, 556. (b) Salaün, J.; Marguerite, J. Org. Synth. Coll. Vol. 1990, 7, 131. (c) Krief, A.; Ronvaux, A.; Tuch, A. Bull. Soc. Chim. Belg. 1997, 106, 699. (d) Krief, A.; Ronvaux, A.; Tuch, A. Tetrahedron 1998, 54, 6903. (e) Shevchuk, T. A.; Kulinkovich, O. G. Russ. J. Org. Chem. 2000, 36, 491; Chem. Abstr. 2000, 133, 362561.
(11) Miesch, M.; Miesch, L.; Horvatovich, P.; Burnouf, D.; Delincée, H.; Hartwig, A.; Raul, F.; Werner, D.; Marchioni, E. Radiat. Phys. Chem. 2002, 65, 233.
(12) Colpaert, J.; Kesteleyn, B.; Boterberg, S.; Stevens, C.; De Kimpe, N. “New Method for the Synthesis of (Z)-2-(5-Tetradecenyl)cyclobutanone as Marker for Irradiated Foodstuffs”, 4th International Conference on Agro and Food Physics, May 16-20, 2000, Instanbul (Turkey), Abstract 11, 74.
(13) Logothetis, L. A. J. Am. Chem. Soc. 1965, 87, 749.
(14) Mino, T.; Masuda, S.; Nishio, M.; Yamashita, M. J. Org. Chem. 1997, 62, 2633.
(15) Bestmann, H. J.; Brosche, T.; Koschatsky, K. H.; Michaelis, K.; Platz, H.; Vostrowsky, O. Tetrahedron Lett. 1980, 21, 747.
(16) Ivanov, I. V.; Groza, N. V. ; Romanov, S. G. ; Kuhn, H. ; Myagkova, G. I. Tetrahedron 2000, 56, 553.
(17) Yadav, J. S.; Nanda, S.; Bhaskar Rao, A. Tetrahedron: Asymmetry 2001, 12, 2129.