
Molecular Diversity Preservation International (MDPI), Sanegergasse
25, Basel CH-4054 Switzerland.
URL http://mdpi.org/lin/, e-mail [email protected]
Abstract: I found earlier that only one solid wedge is enough
for a quadrivalent center (Lin, S. -K. Chirality, 1992, 5,
274). The recent modification of this one-wedge convention will be discussed:
the three normal bonds are imagined as bonds distributed, in a shown order,
on a cone at the opposite side of the solid wedge, regardless of the relative
lengthes of these three normal bonds. This means that representation 4
is correct and has the same configuration as 1-3 and 5.

Keywords: One-wedge convention, stereochemical representation.
Introduction
In the original proposal [1], a restriction is made on the relative lengths of the bonds and the arrangements of these bonds. It is recognized that this restriction is not necessary [2]. We propose that the three normal bonds are imagined as bonds distributed, in a shown order, on an imaginary cone at the opposite side of the solid wedge. All the following drawings (Figure 1) are legal and represent the same configuration. In the original proposal [1], representation 4 is not acceptable. Now the representation 4 is acceptable and has the same configuration as 1-3 and 5.

Figure 1. Acceptable one-wedge sterechemical representations. In the original proposal [1], representation 4 is not acceptable.
Therefore, when drawing a crowded structure, some bonds can be arranged very close to each other, and some bonds may be much longer than the others, and they all still represent the same absolute configuration if the orientation of the solid wedge is the same and the three normal bonds are distributed in the same order.
Proposal of One-Wedge Convention
The solid wedge, broken wedge, broken line, solid bar, and broken bar
are all frequently used for structural drawing (Figure 2). A large number
of their combinations have been seen in literature, which have caused confusion
and ambiguity. It is clear that one type of solid wedge and only one is
enough for a quadrivalent center. One-wedge convention of stereochemical
representation has been introduced [1,2]. Some related recent papers and
background discussions see [1-7] and papers cited therein.
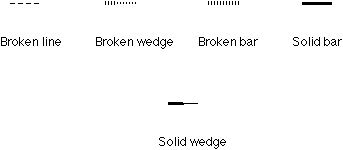
Figure 2. Various bonds used to perspective specification.
For examples, in their recent textbooks, North [8] used a solid wedge and a broken wedge to represent a three dimensional chemical structure, while Eliel and Wilen [9] used a solid wedge and a broken bar.
In 1996 IUPAC published the following recommendation for graphic representation of three-dimensional structures [10]:
Structural diagrams which depict stereochemistry must be prepared with extra care to ensure there is no ambiguity. In general plain lines depict bonds approximately in the plane of the drawing; bonds to atoms above the plane are shown with a bold wedge(starting from an atom in the plane of the drawing at the narrow end of the wedge); and bonds to atoms below the plane are shown with short parallel lines
. As an alternative a bold bond
may be used instead of a bold wedge. A broken line
has been used instead of parallel lines but this is better reserved for a partial bond, delocalisation, or a hydrogen bond. The use of a wedge of parallel lines
is not recommended as it is ambiguous. It is used commonly in two directly opposite ways. Different workers define the narrow end as being in the plane of the drawing or furthest from the viewer. If stereochemistry is unknown this can be indicated explicitly by a wavy line
. The use of dots or open circles at a centre to show stereochemistry is strongly deprecated. Other specific conventions mentioned in the Glossary include Fischer projection, Newman projection, sawhorse projection and wedge projection.
...
With tetrahedral stereochemistry the following are recommended:
In a previous paper [1] we have argued that the broken wedge, broken
line, solid bar, and broken bar are either not reasonable or can be replaced
by only one type of solid wedge in the stereochemical formulation.
We first argues that one solid wedge is enough [1] . Then, a convention
based on a one-wedge symbolization is proposed, where a or b
is used instead of c, d, e, f, g, or
h (Figure 3).

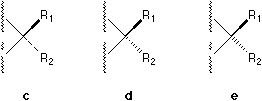

Figure 3. The one-wedge symbolization where a or b is used instead of c, d, e, f, g, or h, etc.
Here, we give the revised convention as follows:
1. One and only one solid wedge (or wedge in the following) is used per center.
2. Only the chiral center concerned is at the plane of the paper. The ligand bearing the solid wedge and the triangle composing the rest three groups, are always at the two opposite sides of the paper.
3. When the wedge points from the ligand to the center, the ligand bearing the solid wedge is above the paper. When the wedge points from the center to the ligand, the ligand bearing the solid wedge is below the paper.
4. The place of the ligand bearing the solid wedge has no influence of the configurations. However, the sense counting along the pericycle of the triangle is important.
5. When only three bonds are drawn, the missing ligand can be an electron pair or a hydrogen, and is eclipsed with the ligand bearing the solid wedge.
In the first proposal, we used a triangle defined by the rest three ligands. Now we understand that this triangle definition is not necessary. There is no influence of the configurations of sense counting along the pericycle of the triangle is important. The sense counting along the rest three bonds already unambiqurisly defined the absolute configuration. Therefore, all the five formulas (1-5) give the same configutation representation.
Calling for Supporters' Signatures
The present author is collecting supporting signatures for the proposal of One-Wedge Convention of Stereochemical Representation. Please send your comments to me by e-mail. Finally I will submit this proposal, together with the signatures of supports, to IUPAC. We also plan to test this convention in the journal Molecules [3].
Acknowledgments: The author thanks Professors Bernard Testa (University of Lausanne) and Miklos Simonyi (Hungarian Academy of Science) for their encouragement.
References and Notes
1. Lin, S. -K. A proposal for the representation of the stereochemistry of quatrivalent centres. Chirality, 1992, 5, 274-278. View this paper in html format (http://www.unibas.ch/mdpi/ecsoc/f0007/f0007lin.htm).
2. (a) Lin, S. -K. One-wedge convention of stereochemical representation; ACS 212th National Meeting, Orlando, Florida, August 25-29, 1996. Download the paper in pdf format at http://www.unibas.ch/mdpi/wedge.pdf. (b) This proposal was also briefly presented at The First Electronic Molecular Modelling & Graphics Society Conference, October 1996. (c) Lin, S. -K. One-wedge convention of stereochemical representation of quadrivalent centers; ECSOC-1, http://www.mdpi.org/ecsoc-1.htm, September 1-30, 1997 (http://www.unibas.ch/mdpi/ecsoc/f0007.htm).
3. This convention may be introduced in the MDPI published Molecules (http://www.mdpi.org/molecules/), the first electronic journal of synthetic chemistry and natural product chemistry.
4. Blessington, B. A serious problem with computer processing of stereochemistry chemical structure files: the need for standardisation. Chirality 1995, 7, 337-341. Dr. Blessington's contact address: Dr.Bernard Blessington . Bradford University . Dept. of Pharmaceutical Chemistry. Bradford BD7 1DP, England. Tel +44 (0) 1274 384704 FAX +44 (0) 1274 305340, e mail - [email protected]
5. Testa, B. On Flying wedges, crashing wedges, and perspective-blind stereochemist. Chirality 1991, 3, 159-160. Professor Testa's contacting address: Prof. Bernard Testa, Institut de Chimie thérapeutique, Section de Pharmacie, Université de Lausanne, CH-1015 Lausanne-Dorigny, Switzerland, Tel. +41 21 692 4521 / 692 4500 , Fax. +41 21 692 4525, Email: [email protected]
6. Maehr, H. A proposed new convention for graphic presentation of molecular geometry and topography. J. Chem. Educ. 1985, 62, 114-120.
7. Xu, J. A proposed new convention for graphic presentation of molecular geometry and topography. J. Chem. Inf. Comput. Sci. 1996, 36, 25-35, particularly pages 31-32.
8. North, M. Principles and Applications of Stereochemistry;
Stanley Thornes: Cheltenham, UK, 1998.
Dr. Michael North has a website for this book at http://www.bangor.ac.uk/ch/mn/booknet/bookhome.htm.
9. Eliel, E. L.; Wilen, S. H. Stereochemistry of Organic Compounds; Wiley: London, 1994.
10. Moss, G. P. IUPAC basic terminology of stereochemistry, recommendations 1996. Pure Appl. Chem., 1996, 68, 2193-2222. See the web version at the http://www.chem.qmw.ac.uk/iupac/stereo/ website, specifically "Graphic Representation of Three-Dimensional Structures" at the http://www.chem.qmw.ac.uk/iupac/stereo/intro.html#gra website. The contact address of Professor Moss: Prof. Dr. G. P. Moss, Department of Chemistry, Queen Mary and Westfield College, Mile End Road, London, E1 4NS, UK, e-mail [email protected]