|
Molbank 2006, M457 |
Synthesis of
1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and 1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene
as heterodienes for iron carbonyl complexes
A. A. Jarrahpour*, A. R. Esmaeilbeig
and A. Adabi Ardekani
Department of Chemistry,
Phone: +98 711 2284822,
Fax: +98 711 2280926 Email: [email protected]
and [email protected]
*Author to whom correspondence
should be addressed
Received: 21 September 2005 / Accepted: 15 January 2006 /
Published: 22 January 2006
Abstract: In this paper we propose the synthesis of
1-(2-aminopyridine)-4-phenyl-1-azabuta-1,3-diene and
1-(3-aminopyridine)-4-phenyl-1-azabuta-1,3-diene as new heterodienes
for iron carbonyl complexes.
Keywords: 2-Aminopyridine, 3-aminopyridine, heterodiene, cinnamaldehyde,
Schiff base.
Introduction
Although a large number
of p- (1,3-diene) iron tricarbonyl
complexes have been reported and their reactivity investigated [1], less is
known of the corresponding heterodiene compounds. In
such compounds, which may be regarded as derived from the basic butadiene unit
by the replacement of one or more of the carbon atoms by the oxygen or
nitrogen, the possibility arises that the lone pair of electrons of the
heteroatom is involved with the metal-ligand bond
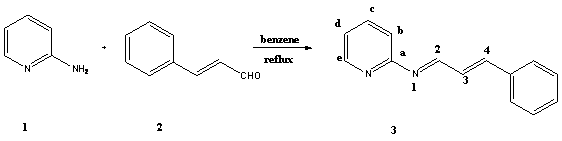
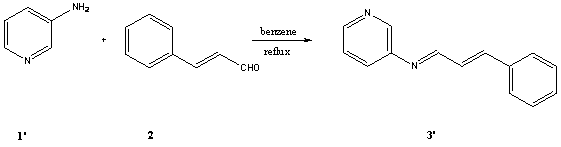
[2-5].In this study we report the synthesis of two new Schiff bases derived
from 2-aminopyridine, 3-aminopyridine and cinnamaldehyde.These
compounds are potentially good ligands for iron
carbonyl complexes.
Results and Discussion
A solution of
2-aminopyridine 1 (0.94g, 10.00mmol) in dry benzene (10mL) was added
with stirring to a solution of cinnamaldehyde 2
(2.64g, 20.00mmol) in benzene (10mL). The reaction mixture was stirred at c.a.
40 ¡ãC for 4 hours. Most of solvent was removed by splash-guard adaptor attached
to vessel. The residue of solvent was removed under reduced pressure. After
returning to room temperature and addition of 100 mL
of n-heptane, the mixture was allowed to stand
overnight in a refrigerator. The upon layer was
extracted and after removal of solvent, the remaining oil was allowed to stand
for 2 days under hood at room temperature. The yellow crystals were separated
by filtration and washed with two 3 mL portions of
cold ether, yielding (1.42g, 68%) of 3. The procedure for preparing 3'
was similar to 3.Compound 3' was a brown solid and its yield was
98%.Its physiochemical properties were almost the same with 3. The IR
spectrum showed the characteristic absorption of Schiff base C=N at 1580 cm-1
and the C=C at 1551 cm-1.. The 1H-NMR
spectrum showed a multiplet
for aromatic protons at 7.20-7.70 p.p.m. He had
a doublet at 9.01 with a J= 8.80. A doublet appeared at 8.46 for H2. H3 and H4
had a multiplet at 6.75. The 13C-NMR
spectrum showed C=N group at 164.20.The mass spectrum showed the (M+1) peak at
209 and the M+at 208.Their ability for
coordination to metal ions are under study.
Melting Point: 68¡ãC.
TLC
(dichloromethane-acetone 20/1): Rf=
0.56
IR (KBr, ¦Í, cm-1): 1580 (s, C=N); 1551 (s, C=C).
1H-NMR (250 MHz, CDCl3): ¦Ä= 9.01 (1H, d, J=8.8, He); 8.46 (1H, d, H2); 7.2-7.7(8H, m, aromatic); 6.75 (2H, m, H3 and
H4).
13C-NMR (62.9 MHz, CDCl3): ¦Ä= 164.2 (C2); 160.7 (Ca); 146.7 (Ce); 148.8 (C3); 146.5 (C4); 138.2 (Cc);
121.9 (Cd); 120.3 (Cb);
127.7-129.9 and 135.5 (Ph carbon atoms).
UV (EtOH; ¦Ëmax nm; ¦Å (dm3.mol-1.cm-1):
319 (18000); 226 (13000).
MS (m/z, %): 209 (M+ +1, 5.9);
208 (M+, 38.3); 207 (M+-1, 50.5); 131 (100); 130
(77); 79 (87.5); 78 (54.2); 52 (49.4); 51 (52.6).
Elemental Analysis: Calculated for C14H12N2: C, 80.77%; H, 5.76%; N, 13.46%. Found:
C, 80.75%; H, 5.89%; N, 13.49%.
Acknowledgment
Authors thank the Shiraz
University Research Council for financial support (Grant No.84-GR-SC-23).
References:
1. Coates, G. E.; Green,
M. L. H.; Wade, K. in Organometallic
Compounds, Vol II,
2. Dieck,
H. T.; Bock, H. Chem. Comm. 1968,
678.
3. Maywald, F.; Eilbracht, P. Synlett. 1996, 380-382.
4. Wrackmeyer, B.; Seidel, G.; Köster, R.Magnetic Resonance in Chemistry, 2000, 38 (7), 520-524.
5. Kuramshin, A. I.; Kuramshina, E. A.; Cherkasov, R. A. Russ. J. Org. Chem., 41 (5), 2005, 649-
655.
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.