|
Molbank 2006, M458 |
Synthesis
of N,N'-bis (a-methylsalicylidene)-3,4'-diaminodiphenyl
ether
A. A. Jarrahpour*, S .Rezaei
Department of Chemistry,
tel: +98 711
2284822, fax: +98 711 2280926, e-mail: [email protected] and [email protected]
*Author
to whom correspondence should be addressed
Received:
Keywords: 2-Hydroxyacetophenone, 3,4'-
diaminodiphenyl ether, Schiff base.
There have been few reports about the
synthesis and application of Schiff-base ligands
derived from 2-hydroxyacetophenone [1].
In order to investigate the electronic, steric and
geometric effect of a methyl group on an imine carbon
on asymmetric catalytic reactions, 2-hydroxyacetophenone (1) has been chosen
as starting material for synthesizing Schiff-base ligands
[2]. Suhai and his coworkers have studied the conformational
effects on the proton affinity of the Schiff base in bacteriorhodopsin
[3]. Jacobsen and his coworkers have studied about asymmetric catalysis
of hetero-ene reaction with tridentate Schiff
base Cr(III) complexes[4].
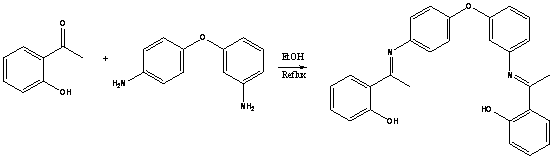
2 3
1
2-Hydroxyacetophenone 1
(0.272 g, 0.24 mL, 2 mmol)
and 3,4'-diaminodiphenylether 2 (0.33 g, 1 mmol) were dissolved in 10 mL of
warm ethanol. The reaction mixture was refluxed for 10h and allowed to stand aside.The solid crystals were filtered off and washed with ethanol.The pure Schiff base 3 was isolated as a
light yellow crystalline solid (yield 78%).
Melting
Point: 182-184 ¡ãC
IR (KBr, ¦Í, cm-1):
3244(OH), 1620(C=N).
1H-NMR (250 MHz, CDCl3): ¦Ä= 1.65(6H, s, CH3),
6.21(2H, d, Ar), 6.24(2H, d, Ar),
6.88-7.64(5H, m, Ar), 7.94(2H, d, Ar),
14.57(2H, s, OH).
13C-NMR (62.9 MHz, CDCl3): ¦Ä= 17.22; 111.71;
113.20; 118.22; 119.63; 126.66; 128.88; 132.95;142.73; 145.60; 146.60; 162.15;
171.23.
MS (m/z): 436 (87%), 317 (40%), 210 (48%), 139
(38%), 91 (90%).
Acknowledgment
The authors thank the Shiraz University Research
Council for financial support (Grant No.84-GR-SC-23)
References:
1. (a) Zhang, J. X.; Zhou, Y.; Cai, G. J. Mol. Catal, 1997, 11, 41-44; (b) Holland, D;
Laidler, D.A.;
Milner, D.J. J. Mol. Catal, 1981, 11,119-127. (c) Chen, G.
M.; Chen,
F.; Zhou, C, Chem.
Chin. Univ, 1995, 16, 216-219. (d) Qiu, M.; Liu, G.; Yiao, X,
Chin. J.Catal, 2001, 22,
77-80. (e) Li, C.; Zhang, W.; Yao, X, Chin. J,
Catal. 2000,
21, 77-80. (f) Li, Z. N.;
Liu, G.; Zheng, Z. Tetrahedron 2000, 56,
7187-7191.
2. Gao, W. T.; Zheng, Z.; Molecules
2002, 7, 511-516.
3. Tajkhorshid,
E.; Paizs, B.; Suhai, S. J.
Phys. Chem. 1997, 101, 8021-8027.
4. Ruck. R. T.; Jacobsen E. J. J. Am. Chem.
Soc. 2002, 124, 2882-2883.
Sample Availability: Available from MDPI.
©
2006 MDPI. All rights reserved.