|
Molbank 2006, M461 |
2-(cyclooct-2-enyloxy)-isoindole-1,3-dione
Sergiu Coseri
Institute of Macromolecular Chemistry ¡°P.Poni¡± Iasi Iasi , Romania
National Research Council of Canada 100
Sussex Dr., Ottawa , ON , K1A
0R6 , Canada
e-mail: [email protected]
Received:
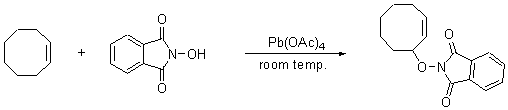
A degassed mixture containing 0.38 mmol cyclooctene and 0.0767 mmol N-hydroxyphthalimide (NHPI),1,2 in 5 mL acetonitrile was added to a degassed solution of lead tetraacetate3 (0.038 mmol in 5 mL acetonitrile) under nitrogen atmosphere, at room temperature. After the completion of the reaction, the solvent and the olefin excess was removed under reduced pressure and the residue was purified by preparative TLC using hexane:ethyl acetate = 2:1 as eluent, giving the above product.
1H-NMR (400 MHz, CDCl3): d=7.84, 7.77 (Ar, 4 H); 5.86, 5.77 (CH=CH, 2H); 5.32 (O-CH, 1H); 2.22, 2.10, 1.67, 1.61, 1.59 (CH2, 10H).
13C-NMR (100 MHz, CDCl3): d= 164.6 (C=O); 134.9, 128.2 (Ar); 85.7 (C-O); 77.6 (C=C); 33.7, 30.5, 28.7, 26.7, 24.6 (CH2).
References
1. Ishii, Y.; Sakaguchi, S.; Iwahama, T., Adv. Synth. Catal., 2001, 343, 393.
2. Masui, M.; Hosomi, K.; Tsuchida, K.; Ozaki, S., Chem. Pharm. Bull., 1985, 33, 4798.
3. Martin, J.C.;
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.