|
Molbank 2006, M462 |
Synthesis and acidic properties of novel
3-methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one
Haydar Y¨¹kseka*, Muzaffer
Alkana and Şule Bahçecib
aEducation
Faculty,
tel: (+90)-474-2126608, fax: (+90)-474-2121185, e-mail: [email protected]
bFatih
Education Faculty,
*Author
to whom correspondence should be addressed
Received:
A number of studies
involving the determination of pKa values of some 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives in non-aqueous
solvents has been revealed [1-3]. 3-Methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one 3 was synthesized from the reaction
of
3-methyl-4-chloroacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one 1 with 2-amino-5-mercapto-1,3,4-thiadiazole
2. Moreover, the
synthesized compound 3 was titrated potentiometrically with tetrabutylammonium
hydroxide (TBAH) in three non-aqueous solvents such as isopropyl alcohol, tert-butyl alcohol and N,N-dimethylformamide to determine pKa
values. For compound 3, the half-neutralization potentials (HNP)
and the corresponding pKa values
were determined in the three non-aqueous solvents mentioned above. The starting compound 1 was prepared
according to literature [2,4].¡¡
3-Methyl-4-chloroacetylamino-4,5-dihydro-1H-1,2,4-triazol-5-one 1 (1.91 g,
0.01 mol) and 2-amino-5-mercapto-1,3,4-thiadiazole 2 (1.33 g, 0.01 mol) in
n-butyl acetate (30 mL) was refluxed for five hours
and then evaporated at 50-55 ¡ãC in vacuo. Several recrystallizations of the residue from ethanol gave pure
compound 3 (1.32 g, 45.99 %).
Melting point: 151-152 ¡ãC (EtOH, uncorrected).
UV (¦Ëmax nm; EtOH) / ¦Å (dm3.mol-1.cm-1): 282
(5980), 203 (9150).
IR (KBr, cm-1):
3400, 3250, 3100 (NH2, NH); 1750 (C=O); 1605 (C=N).
1H-NMR (200 MHz, DMSO-d6): ¦Ä= 2.09
(3H, s, CH3); 4.14 (2H, s, CH2); 6.40 (2H, br, NH2);
11.40 (1H, s, NH); 11.64 (1H, s, NH).
13C-NMR (50 MHz, DMSO-d6): ¦Ä= 10.45
(CH3); 34.75 (CH2); 144.70, 151.76, 152.21, 166.86
(heterocyclic carbons); 169.89 (C=O).
The potentiometric titration curves of 0.001 M compound 3
solutions titrated with 0.05
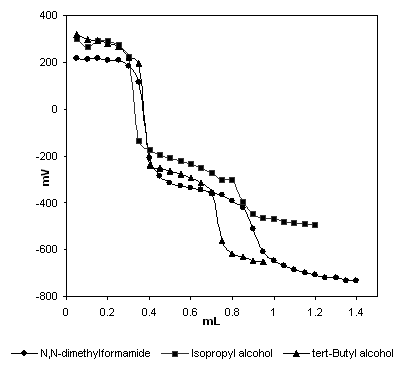
Figure 1
The HNP values and the corresponding pKa values of compound 3 are presented Table 1.
|
Solvent |
HNP1 (mV) |
pKa1 |
HNP2 (mV) |
pKa2 |
|
Isopropyl alcohol |
291 |
2.06 |
-221 |
10.64 |
|
tert-butyl alcohol |
287 |
2.16 |
-269 |
11.61 |
|
N,N-dimethylformamide |
218 |
3.36 |
-342 |
12.68 |
Table1: HNP values and the corresponding pKa values of compound 3
As seen Figure 1 and Table 1,
compound 3 give two end points as well as two half-neutralization
potential values. The potentiometric titration curves
of compound 3 resemble the titration
curves of diprotic acids.
References:
1. H. Y¨¹ksek, Z. Ocak, M. Alkan, Ş. Bahçeci and M. Ozdemir, Molecules., 2004, 9, 232-240.
2. H. Y¨¹ksek, M. Alkan, Z. Ocak, Ş. Bahçeci, M. Ocak and M. Ozdemir, Indian J. Chem., 2004, 43B, 1527-1531.
3. Ş. Bahçeci, H. Y¨¹ksek, Z. Ocak, C. Köksal and M. Ozdemir, Acta Chim. Slov., 2002, 49, 783-794.
4. A.A. Ikizler and R. Un, Chim. Acta Turc., 1979, 7, 269-290; Chem. Abstr. 1981, 94, 15645d.
Sample Availability: Available from the Authors.
© 2006 MDPI. All rights reserved.