|
Molbank 2006, M463 |
Synthesis and acidic properties of new
1-phenylacetyl-3-ethyl-4-(4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one
Haydar Y¨¹kseka*, Muzaffer
Alkana and Şule Bahçecib
aEducation Faculty,
tel: (+90)-474-2126608, fax: (+90)-474-2121185, e-mail: [email protected]
bFatih
Education Faculty,
*Author
to whom correspondence should be addressed
Received:
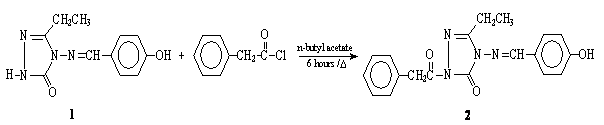
It is known that 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one rings have weak acidic properties, so some 1,2,4-triazole and 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives were titrated potentiometrically with tetrabutylammonium hydroxide in non-aqueous solvents, and the pKa values of the compounds were determined [1-3]. Determination of the pKa values of active constituent of certain pharmaceutical preparations is important, because their distribution, transport behavior, bonding to receptors, and contributions to metabolic behavior of the active constituent molecules depend on the ionization constant [4]. 1-Phenylacetyl-3-ethyl-4-(4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one 2 was synthesized from the reaction of 3-ethyl-4-(4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one 1 with phenylacetyl chloride. Moreover, the synthesized compound 3 was titrated potentiometrically with tetrabutylammonium hydroxide (TBAH) in four non-aqueous solvents such as isopropyl alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethylformamide to determine pKa values. For compound 2, the half-neutralization potentials (HNP) and the corresponding pKa values were determined in the four non-aqueous solvents mentioned above. The starting compound 1 was prepared according to literature [5].¡¡
3-Ethyl-4-(4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one 1 (2.32 g,
0.01 mol) was refluxed with a solution of phenylacetyl
chloride (0.01 mol) in n-butyl acetate (40 mL) for 6
h and then allowed to cool. The crystals formed were filtered. The product was recrystallized from EtOH-H2O (1:3) gave pure
compound 2 (1.62 g, 46.0 %).
Melting point: 200-201 ¡ãC
(EtOH-H2O, 1:3; uncorrected).
UV (¦Ëmax nm; EtOH) / ¦Å (dm3.mol-1.cm-1) 310
(13720); 222 (12290); 207 (14100).
IR (KBr, cm-1):
3500 (OH); 1760 (C=O); 1610, 1585 (C=N); 850 (1,4-disubstituted benzenoid
ring); 735, 695 (monosubstituted benzenoid ring).
1H-NMR (200 MHz, DMSO-d6): ¦Ä= 1,24
(3H, t, CH3); 2.72 (2H, q, CH2); 4.27 (2H, s, CH2);
6.90 (2H, d, Ar-H, J=7.02 Hz); 7.33 (5H,
s, Ar-H); 7.70 (2H, d, Ar-H, J=7.02
Hz); 9.39 (1H, s, N=CH); 10.32 (1H, s, OH).
13C-NMR (50 MHz, DMSO-d6): ¦Ä= 9.40 (CH3); 18.80 (CH2); 41.20 (CH2Ph); 116.05 (2C); 123.80, 126.90, 128.41 (2C), 129.99 (2C); 130.17 (2C); 134.00, 161.40 (aromatic carbons); 148.20 (triazole C3); 150.40 (N=CH); 157.00 (triazole C5); 167.30 (C=O).
Elemental
Analysis: Calculated for C19H18N4O3
(350.38): C, 65.13%; H, 5.18%; N, 15.99%. Found: C, 64.89%; H, 4.47%; N,
15.82%.
The HNP values and the
corresponding pKa values of compound 2 in isopropyl
alcohol, tert-butyl alcohol, acetonitrile and N,N-dimethylformamide
were: -275 mV (11.40), -374 mV (13.01), -392 mV (13.23) and -468 mV (-),
respectively. The pKa value has not
been obtained in N,N-dimethylformamide.
References:
1. H. Y¨¹ksek, Z. Ocak, M. Ozdemir, M. Ocak, M. Bekar and
M. Aksoy,
Indian J. Heterocycl.
Chem., 2003, 13,
49-52.
2. H. Y¨¹ksek, M. Alkan, Z. Ocak, Ş. Bahçeci, M. Ocak and M. Ozdemir, Indian J. Chem., 2004, 43B, 1527-1531.
3. Ş.
Bahçeci, H. Y¨¹ksek, Z. Ocak,
4. A. Demirbaş, I. Kula, Y. Erdoğan, A. Aslan, N. Yaylı and S. Karslıoğlu, Energy Edu. Sci. Tech., 1998, 1, 1-6.
5. H. Y¨¹ksek, M. K¨¹ç¨¹k, M. Alkan, Ş.
Bahçeci, S. Kolaylı, Z. Ocak, U. Ocak, E. Şahinbaş and M. Ocak, Asian J. Chem., (in press).
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.