|
Molbank 2006, M471 |
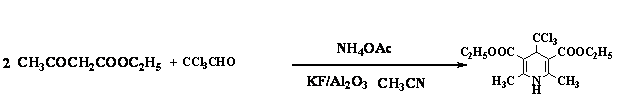
Diethyl 2,6-Dimethyl,4-(1,1,1-trichloromethyl)-1,4-dihydropyridine-3,5-dicarboxylate
Fatma Aydina* and Recep Ozenb
aCanakkale Onsekiz Mart University TURKEY
Tel: +90 286 2180018-1862, Fax: +90 286 2180533 e-mail: [email protected]
*Author to whom correspondence should be addressed
Received:
Keywords: Hantzsch reaction, 1,4-dihydropyridines,
chloral, KF/Al2O3
In 1882, Hantzsch reported the
first synthesis of dialkyl
1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylates from a refluxing mixture of
an aldehyde, a ¦Â-ketoester,
and aqueous ammonium hydroxide in ethanol.[1] 1,4-dihydropyridines (1,4-DHPs)
are well known as Ca+2 channel blockers.[2,3]
Concentric H2SO4
(5 mL) was dropped on chloral hydrate (3 g) and 1,1,1-trichloroacetaldehyde was distilled at 98oC.
Acetylacetone (4 mL, 40 mmol), freshly distilled 1,1,1-trichloroacetaldehyde
(2.94 g, 20 mmol) and CH3COONH4
(1.44 g, 20 mmol) were dissolved in acetonitrile (15 mL). This
mixture was added on [4] and refluxed for 3 h. The progress of the reaction was
monitored by TLC analysis. After completion of the reaction, the resulting
suspension was filtered and solid washed with acetonitrile
(5 mL), solvent was evaporated. Residue was washed
with water and extracted in CH2Cl2 (3x5 mL). The solution of CH2Cl2 was dried
over anhydrous Na2SO4 and then filtered..
The solvent was removed under reduced pressure and pale yellow oil product in a good yield, 67.1 % (4.97 g).
UV ¦Ëmax (nm;
ethyl alcohol) / ¦Å (dm3.mol-1.cm-1): 392 / 6270
IR (KBr) (¦Ô cm-1): 3419 (N-H); 2984 (C=C); 1722 (C=C-C=O); 1031 (C-O); 818 (C-Cl).
1H-NMR (400 MHz, CDCl3): ¦Ä= 1.21 (t, 6H, -CH3); 2.16 (s, 6H, -CH3); 3.31 (s, 1H); 4.12 (q, 4H, methylenic).
13C-NMR (100 MHz, CDCl3): ¦Ä= 29.8,
31.6, 58.5, 62.0, 81.7, 166.7, 169.0, 199.5.
Elemental Analysis: Calculated for C14H18 Cl3NO4:
C, 45.37%; H, 4.89%; Cl, 28.69%; N, 3.78%. Found: C,
45.21%; H, 4.84%; Cl, 28.72%; N, 3.82%.
References
1.
A. Hantzsch, Ann. 215,
1, 72 (1882); Ber. 18 1744 (1885);
19, 289 (1886).
2.
a) Bossert, F.; Meyer,
H.; Wehinger, E. Angew.
Chem., Int. Ed. Engl. 1981, 20, 762-769;
b) Nakayama, H.; Kasoaka,
Y. Heterocycles 1996, 42, 901-909.
3.
Love, B.; Goodman, M.; Snader,
K.; Tedeschi, R.; Macko, E.
J. Med. Chem. 1974, 17, 956-965.
4.
Aydin, F. and Ozen, R. J. Chem. Res-S, (7), 2004,
486-487.
5.
Younessi, A. and Krapivin, G. D., Molbank, 2003, M344
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights
reserved.