Molbank 2006,
M474
http://www.mdpi.org/molbank/
5,5’-Biindole
Ramiro
Quintanilla-Licea 1.*, Juan F.
Colunga-Valladares 2, Adolfo Caballero-Quintero 3,
Noemí
Waksman 3, Ricardo Gomez-Flores 1, Cristina
Rodríguez-Padilla
1 and Reyes Tamez-Guerra 1
1 Facultad de Ciencias
Biológicas, 2
Facultad de Ciencias Químicas and 3 Facultad de
Medicina,
Universidad
Autónoma de Nuevo León, Pedro de Alba s/n,
Cd. Universitaria,
San Nicolás de los Garza, Nuevo León, México
* Author to whom
correspondence should be addressed;
E-mail: [email protected]
Received for
MOLECULES:
Abstract:
Synthesis of 5,5’-biindole was carried out by the Madelung indole
reaction.
Under strong basic conditions and high temperatures (350 ºC), N,N’-bis-formyl-o-tolidine
underwent cyclization to produce high amounts of the dimeric indole.
Full and
unambiguous assignments of all 1H- and 13C-NMR
resonances
of indole and 5,5’-biindole in DMSO-d6 are also
reported.
Keywords: Indoloquinolizine,
Antitumor activity, Teuber’s reaction, Indole synthesis, 1H-NMR,
13C-NMR.
Introduction
Scheme 1.
Naturally-occurring indoloquinolizines.
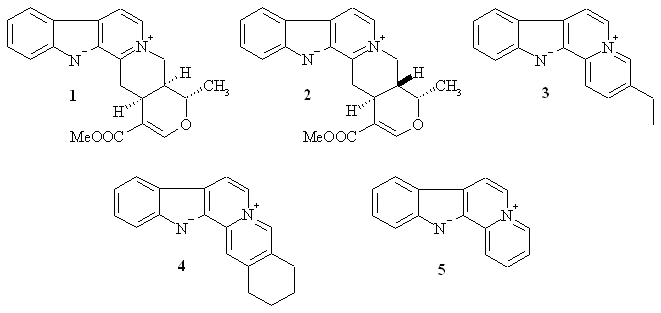
Natural
indoloquinolizines have been
reported to inhibit DNA synthesis in cancer cell lines [14-16]. In this
regard,
alstonine 1, serpentine 2 and sempervirine 4
(Scheme 1),
were shown to protect BALB/c and Swiss mice from cancer induced by YC8
lymphoma
and Ehrlich carcinoma cells, respectively [15]. In addition, it was
reported
that synthetic derivatives of the natural product javacarboline showed
potent
antitumor activities against P-388 murine leukemia cells and PC-6 human
lung
carcinoma cells [16].
Since there are
few and complex
strategies available to obtain the indoloquinolizine basic tetracyclic
structure [17], we are developing Teuber’s reaction [18] as a useful
and easy
method for obtaining synthetic indoloquinolizines, as new antitumor
agents,
from tryptamines and b–dicarbonyl
compounds. Thus, starting with tryptamine
hydrochloride 6 and acetylacetaldehyde dimethyl acetal, Teuber et
al.
obtained 3-acetyl-7,12-dihydro-2-methyl-6H-indolo[2,3-a]quinolizinium
chloride 7, which after oxidation with o-chloranyl
yielded the compound 8 with ring C of the tetracyclic
system completely aromatized;
treatment of 8 with base yielded the corresponding
indoloquinolizine 9
(Scheme 2) [19, 20].
Scheme 2.
Teuber’s reaction for synthesis of indoloquinolizines.
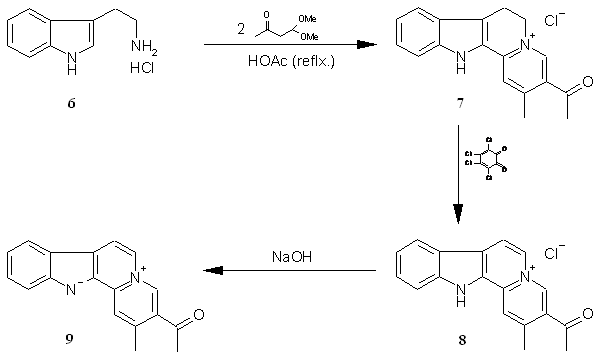
Recently,
Solís-Maldonado et al.
[21] demonstrated that compounds 7-9 possessed
differential
effects on in vitro rat lymphocyte and macrophage
functions;
proliferation of thymic lymphocytes was significantly (p<0.05)
increased (up to 30% increase) by compound 7 at concentrations
ranging
from 10-11 to 10-5 M, compared with untreated
control. In
addition, tumor necrosis factor-a and
nitric acid production by peritoneal macrophages was significantly (p<0.05)
increased (up to 30% increase) by compounds 8 and 9
(Scheme 2) at
concentrations of 10-11 to 10-5 M, and 10-5
M,
respectively. The dimeric indoloquinolizine 10 (Scheme 3),
obtained by
reaction of dihydroindoloquinolizine 7 (Scheme 2) with
benzaldehyde
under piperidine catalysis [20], showed higher effects (up to 40%
increase) on
lymphocytes proliferation than the monomeric ones [21].
Scheme
3.
Synthesis of dimeric indoloquinolizine 10.

These synthetic indoloquinolizines, particularly the dimeric compounds, could serve then as immunotherapeutic agents by selectively increasing the pool of activated T lymphocytes or stimulating macrophage functions, with potential use in treatment of infectious diseases and cancer.
Scheme 4.
Retrosynthesis of dimeric indoloquinolizine 11.
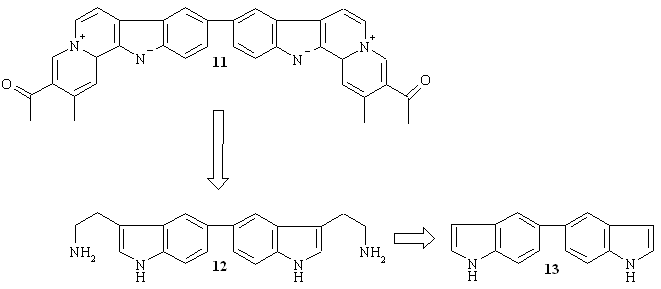
Results and Discussion
The Madelung
indole synthesis is a
method for producing indoles from a base-catalyzed thermal cyclization
of N-acyl-o-toluidides
[31, 32], and is one of the few known reactions by which the simple
indole
compound 15 (Scheme 5) can be obtained [33-35].
The most usual
conditions previously
reported by others include sodium or potassium alkoxide at temperatures
of
200-400 ºC; thus, indole 15 (79% yield) can be obtained from N-formyl-o-toluidine
14 (Scheme 5) [36-38]. This yield is calculated on the
assumption that 2
moles of N-formyl-o-toluidine 14 are required
for the
production of 1 mole of indole [33], although the mechanism of
Madelung´s
reaction has not been completely elucidated [32].
Scheme 5.
Madelung´s indole reaction.
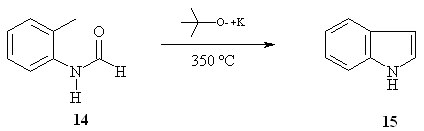
Therefore we
decided to use the Madelung´s indole reaction to synthesize
5,5´-biindole 13
starting from N,N’-bis-formyl-o-tolidine 16
(Scheme
6) [36].
Scheme 6. Synthesis of 5,5´-biindole 13.
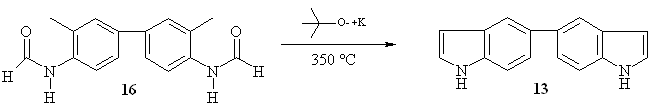
After work-up, we
obtained a brown
oil which crystallized on cooling. The yield was 1.9 g (88%).
Purification by
recrystallization in chloroform led to a light-brown solid with a
melting point
of 195 ºC. The ESMS showed a molecular ion at 233
1H-1H-COSY,
HMQC and HMBC experiments established geminal and vicinal hydrogen
interactions, as well as direct (1JCH) and
two and
three bond correlations between carbon and hydrogen in the structure of
both
indole 15 and 5,5’-biindole 13. The definite assignment
of the
chemical shifts of protons and carbons are shown in Tables 1 and 2.
Table 1. 1H(400
MHz) and 13C (100MHz)
NMR spectral data for indole 15 in DMSO-d6,
including
results obtained by heteronuclear 2D shift-correlated HMQC (1JCH)
and HMBC (nJCH, n=2 and 3). Chemical
shifts (d, ppm) and coupling constants (J, Hz, in
parenthesis).a
POSITION
|
dH |
dC |
bDEPT 135 |
COSY 1H-1HCorrelations |
cHMQC
1JCH |
cHMBC (12Hz)
2JCH
3JCH |
|
|
11.08 (s) |
|
|
H-2 |
|
|
|
|
|
2 |
7.33 (t, 2.65) |
125.07 |
(+) CH |
N-H, H-3 |
H-2 |
H-3 |
|
|
3 |
6.42 (d, 2.65) |
100.84 |
(+) CH |
H-2, H-4 |
H-3 |
H-2 |
|
|
3a |
|
127.47 |
(0) Cq |
|
|
H-3 |
H-2, H-5, H-7 |
|
4 |
7.53 (d, 7.82) |
119.87 |
(+) CH |
H-5 |
H-4 |
|
H-6 |
Table
1.
Cont.
|
5 |
6.98 (t, 7.37) |
118.64 |
(+) CH |
H-4 |
H-5 |
|
H-7 |
|
6 |
7.07 (t, 7.52) |
120.75 |
(+) CH |
H-7 |
H-6 |
|
H-4 |
|
7 |
7.39 (d, 8.06) |
111.26 |
(+) CH |
H-6 |
H-7 |
|
H-5 |
|
7a |
|
135.71 |
(0) Cq |
|
|
|
H-2, H-3, H-4, H-6 |
a)
Number
of hydrogens bound to carbon atoms deduced by comparative analysis
of DEPT 135-13C-NMR spectra. Chemical shifts and coupling
constants
(J) obtained of 1D 1H-NMR spectrum
b)
DEPT
shows CH, CH2, CH3, Cq
c)
Correlation
from C to the indicated hydrogens
1D NOE difference
measurements
established the signal of proton 7 of indole in the 1H-NMR
spectrum,
since NOEs between the doublet at 7.39 ppm and the singlet at 11.08 ppm
(N-H)
could be detected. Further NOEs between resonances at 11.08 and 7.33
ppm
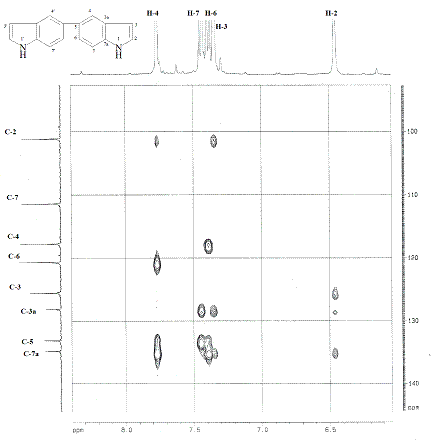
demonstrated the vicinity of N-H and H-2. In the HMBC spectrum of
indole
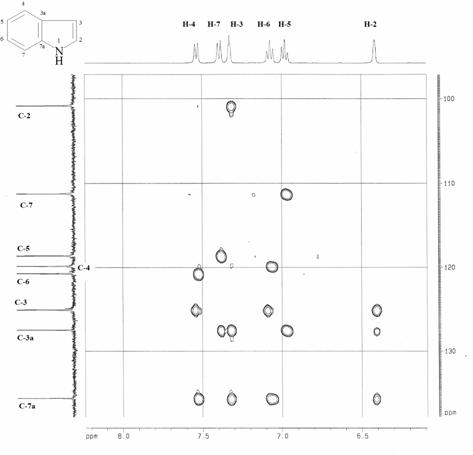
(Figure 1) three bond connectivity between C-7 and H-5 can be observed,
whereas
in HMBC spectrum of 5,5’-biindole (Figure 2) this is absent, indicating
the
joint point of the two indole nuclei.
Table 2. 1H(400
MHz) and 13C (100MHz)
NMR spectral data for 5,5’-biindole 13 in DMSO-d6,
including results obtained by heteronuclear 2D shift correlated HMQC (1JCH)
and HMBC (nJCH, n=2 and 3). Chemical
shifts (d, ppm) and coupling constants (J, Hz, in
parenthesis).a
bPOSITION
|
dH |
dC |
cDEPT 135 |
COSY 1H-1HCorrelations |
dHMQC
1JCH |
dHMBC (12Hz)
2JCH
3JCH |
|
|
1 (N-H) |
11.06 (s) |
|
|
H-2, H-3 |
|
|
|
|
2 |
7.34 (s) |
125.59 |
(+) CH |
N-H, H-3 |
H-2 |
H-3 |
|
|
3 |
6.46 (s) |
101.22 |
(+) CH |
H-2 |
H-3 |
H-2 |
H-4 |
|
3a |
|
128.18 |
(0) Cq |
|
|
H-3 |
H-2, H-7 |
|
4 |
7.77 (s) |
117.81 |
(+) CH |
|
H-4 |
|
H-6 |
|
5 |
|
133.12 |
(0) Cq |
|
|
|
H-7 |
|
6 |
7.39 (d, 8.42) |
120.72 |
(+) CH |
H-7 |
H-6 |
|
H-4 |
|
7 |
7.44 (d, 8.38) |
111.45 |
(+) CH |
H-6 |
H-7 |
|
|
|
7a |
|
134.80 |
(0) Cq |
|
|
|
H-2, H-3, H-4, H-6 |
a)
Number
of hydrogens bound to carbon atoms deduced by comparative
analysis of DEPT 135-13C-NMR spectra. Chemical shifts and
coupling
constants (J) obtained of 1D 1H NMR spectrum
b)
Corresponding
also to positions 1’-7a’ due to symmetry of the molecule
c)
DEPT
shows CH, CH2, CH3, Cq
d)
Correlation
from C to the indicated hydrogens
Figure
2.
HMBC spectra of 5,5’-biindole 13.
Conclusions
Among several
methods available for
the synthesis of biindole compounds [44-47], the use of the Madelung´s
indole
reaction was found suitable for the cyclization of N,N’-bis-formyl-o-tolidine
to produce 5,5’-biindole.
Acknowledgments
The authors would
like to thank the
Deutscher Akademischer Austauschdienst (DAAD, Germany), who financed a
2-month
stay of R. Quintanilla-Licea at the University of Göttingen. We deeply
appreciate Prof. Lutz F. Tietze of the University of Göttingen for
supporting
us with MS measurements and SDBSWeb (http://www.aist.go.jp/RIODB/SDBS)
for free access to its spectral data bank (19 January 2006). This study
was
supported by PAICYT grants CN892-04 and CN1097-05 from the Universidad
Autónoma
de Nuevo León (Mexico) to RQL.
Experimental
General
NMR spectra were
recorded in DMSO-d6
at 25 ºC on a spectrometer Bruker DPX400 operating at 400.13 MHz
for 1H,
and 100.61 MHz for 13C. Chemical shifts are given in ppm
relative to
TMS. Thin layer chromatography (TLC) was performed on precoated plates
(Aldrich
TLC aluminum sheets silica 60 F254) with detection by UV
light. FTIR
spectra were taken on a Perkin-Elmer spectrometer using potassium
bromide
pellets. Mass spectra were measured with a Varian MAT 311A
Spectrometer.
Melting points were determined on an Electrothermal 9100 apparatus and
are
uncorrected. Indole was obtained from Sigma-Aldrich (St. Louis, MO).
5,5’-Biindole (13).
A 500 mL
three-necked round-bottomed
flask is fitted with a reflux condenser and a gas inlet tube connected
to a
cylinder of nitrogen. The third opening of the flask is closed by a
stopper.
The top of the condenser is connected to an air trap which consists of
two 250
mL suction flasks connected in series (the first one is empty; the
second one
contains paraffin oil, and the inlet tube of this flask extends
slightly below
the surface of the oil). In the reaction flask is placed tert-butyl
alcohol (150 mL) and the air in the flask is displaced by dry nitrogen
gas.
Then metallic potassium (4 g, 0.1 mol) is added, in portions, to the
alcohol.
The mixture is heated gently until all potassium has dissolved, and
then N,N’-bis-formyl-o-tolidine
16 (5 g, 0.019 mol) [36] is added and brought into solution. The
condenser is set for distillation with a filter flask as the receiver;
this
flask is protected from the air by connecting it to the trap used in
the
initial operation. The reaction flask is surrounded by an electric
mantle, and
the excess alcohol is removed by distillation. The residue is heated to
350-360
ºC for about 30 minutes and then is allowed to cool in a stream of
nitrogen.
The residue is decomposed by addition of water (100 mL). The mixture is
extracted
successively with chloroform (100 and 50 mL), and the combined
chloroform
extracts are shaken with cold dilute 5% hydrochloric acid. The
chloroform
extract is washed with water (50 mL) of, followed by 5% sodium
carbonate
solution (50 mL), and is dried over anhydrous sodium sulfate and the
solvent
was evaporated. A brown oil was obtained which crystallized on cooling.
The
yield was 1.9 g (88%). Purification by recrystallization in chloroform
led to a
light-brown solid with a melting point of 195 ºC. TLC: Rf =
0.6
(hexane-acetone, 3:2); IR (KBr): vmax 3406,
1625, 1469,
1406, 1343, 749 cm-1; ESMS (positive ion mode): m/z
233 ([M+H].+, base peak; Calcd. for C16H12N2,
232). 1H-NMR and 13C-NMR see Table 2.
References
1.
Sundberg, R.J. The
chemistry of indoles. In Organic
Chemistry, a series of monographs; Blomquist, A.T., Editor;
Academic Press:
New York and London, 1970.
2.
Saxton, J.E.; Indoles,
Part four, The
monoterpenoid
indole alkaloids. In The Chemistry of Heterocyclic
Compounds, a
series of monographs; Weissberger, A. and Taylor, E.C., Editors;
John Wiley
& Sons: New York, 1983.
3.
Hesse, M. Alkaloids,
Nature’s curse or blessing?;
VHCA: Zürich, 2002.
4.
Monien, B.H.;
Krishnasamy, C.; Olson, S.T.; Desai, U.R. Importance of
Tryptophan 49 of Antithrombin in Heparin Binding and Conformational
Activation. Biochemistry 2005, 44, 11660-11668.
5.
Tafazoli, S.;
O'Brien, P.J. Prooxidant
Activity and Cytotoxic Effects of Indole-3-acetic Acid Derivative
Radicals. Chem. Res.
Toxicol. 2004, 17, 1350-1355.
6.
Wang, G.; Geng,
L. Statistical and Generalized Two-Dimensional
Correlation Spectroscopy of Multiple Ionization States. Fluorescence of
Neurotransmitter Serotonin. Anal. Chem. 2005, 77, 20-29.
7.
Fronza, G.; Mele,
A.; Redenti, E.; Ventura, P. 1H NMR and
Molecular Modeling Study on the Inclusion Complex b-Cyclodextrin-Indomethacin.
J. Org. Chem. 1996,
61, 909-914.
8.
Bontchev, P.R.;
Pantcheva, I.N.; Bontchev, R.P.;
Ivanov, D.S.; Danchev, N.D. Copper (II) Complexes of the b-blocker
pindolol: properties, structure, biological activity. BioMetals
2002,
15, 79-86.
9.
Yokoshima, S.;
Ueda T.; Kobayashi, S.; Sato, A.;
Kuboyama, T.; Yokuyama, H.; Fukuyama, T. Sterocontrolled Total
Synthesis of
(+)-Vinblastine. J. Am. Chem. Soc. 2002, 124,
2137-2139.
10.
Li,
V.-S.; Reed, M.; Zheng, Y.; Kohn, H.; Tang, M.-s. C5 Cytosine
Methylation at
CpG Sites Enhances Sequence Selectivity of Mitomycin C-DNA Bonding. Biochemistry
2000, 39, 2612-2618.
11.
Kirsch,
G.H. Heterocyclic Analogues of
Carbazole Alkaloids. Curr. Org. Chem. 2001, 5, 507-518.
12.
Kamijo,
S.; Yamamoto, Y. A Bimetallic Catalyst and Dual Role Catalyst:
Synthesis of N-(Alkoxycarbonyl)indoles
from (2-(Alkynyl)phenylisocianates. J. Org. Chem. 2003,
68,
4764-4771.
13.
Stöckigt,
J. Biosynthesis in Rauwolfia serpentine – Modern
Aspects of an
Old Medicinal Plant. The Alkaloids 1995, 47,
115-172.
14.
Beljanski,
M.; Beljanski, M.S. Selective Inhibition of in vitro Synthesis of
cancer DNA by
Alkaloids of b-Carboline
Class. Exp. Cell Biol. 1982, 50, 79-87.
15.
Beljanski,
M.; Beljanski, M.S. Three Alkaloids as Selective Destroyers of Cancer
Cells in
Mice. Oncology 1986, 49, 198-203.
16.
Yoshino,
H.; Koike, K.; Nikaido T. Synthesis and Antitumor Activity of
Javacarboline
Derivatives. Heterocycles 1999, 51, 281-293.
17.
Matia
M.P.; Ezquerra, J., García-Navio; J.L.; Vaquero, J.J.; Alvarez-Builla,
J. New
Uses of Westphal Condensation: Synthesis of Flavocorylene and Related
Indolo[2,3-a]quinolizinium Salts. Tetrahedron Lett. 1991,
32,
7575.
18.
Teuber,
H.J.; Hochmuth, U. Eine einfache Indolo[2,3-a]chinolizin-Synthese.
Zugleich eine Modell-Reaktion für die Alkaloid-Biogenese? Tetrahedron
Lett. 1964, 325-329.
19.
Teuber,
H.J., Quintanilla-Licea, R.; Raabe T. Indolo[2,3-a]quinolizines
and a
Convenient Synthesis of Flavoserpentine. Liebigs Ann. Chem.
1988, 1111-1120.
20.
Quintanilla-Licea,
R., Teuber, H.J. Review on reactions of acetylacetaldehyde with
aromatic amines
and indoles – Synthesis of heterocycles via hydroxymethylene
ketones. Heterocycles
2001, 55, 1365.
21.
Solís-Maldonado,
C.; Quintanilla-Licea, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.;
Gomez-Flores, R. Differential Effects of synthetic indoloquinolizines
on in
vitro Rat Lymphocyte and Macrophage Functions. Int. Immunopharmacol.
2003,
3, 1261-1271.
22.
von Bayer, A. Ann.
Chem. 1866, 140,
295.
23.
Remers,
W.A.; Brown, R.K. In Indoles, Part One; Houlihan, W.J., Ed.;
John Wiley
& Sons, Inc.: New York, 1972.
24.
Kuethe,
J.T.; Wong, A.; Davies, I.W. Rapid and Efficient Synthesis of 1H-Indol-2-yl-1H-quinolin-2-ones.
Org. Lett. 2003, 5, 3975-3978.
25.
Pal,
M.; Dakarapu, R.; Padakanti, S. A direct Access to
3-(2-Oxoalkyl)indoles via Aluminum
Chloride Induced C-C Bond Formation. J. Org. Chem. 2004,
69,
2913-2916.
26.
Szántay,
C. Synthetic Studies in Alkaloid Chemistry. The Alkaloids 1998,
50,
377-414.
27.
Takao,
K.-i.; Munakata, R., Tadano, K.-i. Recent Advances in Natural Product
Synthesis
by Using Intramolecular Dies-Alder Reactions. Chem. Rev. 2005,
105,
4779-4807.
28.
Babu,
G.; Orita, A.; Otera, J. Novel Synthesis of 2-Aryl and
2,3-Disubstituted
Indoles by Modified Double Elimination Protocol. Org. Lett. 2005,
7, 4641-4643.
29.
Peters
R.; Waldmeier, P.; Joncour, A. Efficient Synthesis of a 5-HT2C
Receptor Agonist Precursor. Org. Process Res. Dev. 2005,
9,
508-512.
30.
Dalpozzo R.;
Bartoli G. Bartoli Indole Synthesis. Curr. Org. Chem. 2005,
9, 163-178.
31.
Madelung,
W. Chem. Ber. 1912, 45, 1128.
32.
Houlihan,
W.J.; Parrino, V.A.; Uike, Y. Lithiation of N-(2-Alkylphenyl)alkanamides
and Related Compounds. A Modified Madelung Indole Synthesis. J.
Org. Chem.
1981, 46, 4511-4515.
33.
Tyson,
F.T. Indole Preparation. J. Am. Chem. Soc. 1941, 63,
2024-2025.
34.
Galat,
A.; Friedman, H.L. Indole from Formyl-toluidine. J. Am. Chem. Soc.
1948,
70, 1280-1281.
35.
Tyson,
F.T. Preparation of Indole. J. Am. Chem. Soc. 1950, 72,
2801-2803.
36.
Quintanilla-Licea,
R.; Colunga-Valladares, J.F., Caballero-Quintero A., Rodríguez-Padilla,
C.;
Tamez-Guerra, R.; Gomez-Flores, R.; Waksman, N. NMR Detection of
Isomers
Arising from Restricted Rotation of C-N Amide Bond of N-formyl-o-toluidine
and N,N’-bis-formyl-o-tolidine. Molecules
2002,
7, 662-673.
37.
Vogel,
A.I.; Furniss, B.S. Vogel’s Textbook of
Practical Organic Chemistry, Including Qualitative Organic Analysis;
Longman: London and New York, 1978,
pp.885-886.
38.
Tyson, F.T.
Indole. Org. Synth. Coll. Vol. III 1955,
479-482.
39.
Hiremat,
S.P., Hosmane, R.S. Applications of Nuclear Magnetic Resonance
Spectroscopy to
Heterocyclic Chemistry: Indole and its Derivatives. In Advances in
Heterocyclic Chemistry. Katritzky, A.R. and Boulton, A.J., Eds.;
Academic
Press: New York and London, 1973;
Vol. 15, pp. 278-324.
40.
Crabb,
T.A. Nuclear Magnetic Resonance of Alkaloids. In Annual Reports on
NMR
Spectroscopy. Webb, G.A., Ed.; Academic Press: London, 1978;
Vol. 8, pp. 2-198.
41.
Ernst,
L.; Kang, S. Carbon-13 NMR Spectroscopy of Substituted Indoles and
Tryptamines.
J. Chem. Res. (S) 1981, 259; J. Chem. Res. (M) 1981,
3019-3027.
42.
Integrated
Spectral Data Base System for Organic compounds. http://www.aist.go.jp/RIODB/SDBS;
[a] SDBS No. 717HSP-49-404; [b] SDBS No. 717CDS-00-313.
43.
Beller,
M.; Breindl, C.; Riermeier, T.H.; Tillack, A. Synthesis of
2,3-Dihydroindoles,
Indoles, and Anilines by Transition Metal-Free Amination of Aryl
chlorides. J.
Org. Chem. 2001, 66, 1403-1412.
44.
Gossler,
H; Plieninger, H. Indoles by Reduction of Isatines, II – Synthesis and
Properties of Biindoles. Liebigs Ann. Chem. 1977,
1953-1958.
45.
Suvorov,
N.N.; Samsoniya, Sh.A.; Chilikin, L.G.; Chikvadize, I.Sh.; Turchin,
K.F.;
Efimova, T.K.; Tret’yakova, L.G.; Gverdtsiteli, I.M. Bisindoles. I.
Synthesis
of 3,5’- and 5,5’-bis-1H-indoles. Khim. Geterotsikl.
Soedin.
1978, 217-224.
46.
Rollin,
Y.; Troupel, M.; Tuck, D. G.; Perichon, J. The Coupling of Organic
Groups by
the Electrochemical Reduction of Organic Halides: Catalysis by
2,2’-bipyridinenickel Complexes. J. Organomet. Chem. 1986,
303,
131-137.
47.
Courtois,
V.; Barhdadi, R.; Troupel, M. Perichon, J. Electroreductive Coupling of
Organic
Halides in Alcoholic Solvents. An Example: the Electrosynthesis of
Biaryls
Catalyzed by Nickel-2,2’-bipyridine Complex. Tetrahedron 1997,
53,
11569-11576.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.