|
Molbank 2006, M483 |
N,N-bis[(1,5-dimethylpyrazol-3-yl)methyl]para-toluidine
Ibrahim Bouabdallah*,
Abdelkrim Ramdani, Ismail Zidane and Rachid Touzani
Laboratoire de Chimie
Organique Physique, D¨¦partement
de Chimie, Facult¨¦ des
Sciences, Universit¨¦ Mohamed Premier, BP 524, 60000,
Oujda, Maroc
e-mail: [email protected]
*Author to whom correspondence should
be addressed
Received:
Keywords : Tripod, pyrazol, ligand, tridentate.
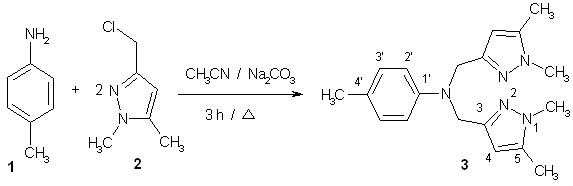
The mixture of para-toluidine 1 (0.535 g, 5 mmol), 3-chloromethyl-1,5-dimethylpyrazol 2 (1.44 g, 10 mmol) and sodium carbonate (4.24 g, 40 mmol) in acetonitrile (50 mL) was refluxed for three hours [1, 2, 3]. The solvent was removed at reduced pressure. The residue was purified through a silica column (CH2Cl2) to give the product 3 (726 mg, 45 % yield) as a white solid.
Melting point: 98 - 100¡ãC (CCl4)
IR (KBr, cm-1): 3020 (¦Í=C-H); 2960 (¦ÍC-H, CH3); 2920 (¦ÍC-H, CH2); 2875 (¦ÍC-H, N-CH3); 1620 (¦ÍC=N); 1530 (¦ÍC=C); 1430; 1390 (¦ÄC-N);
1340; 1235; 1185; 940; 800 (¦Ä=C-H); 780.
1H-NMR (CDCl3, 300 MHz): d= 6.94 (d, 2 H, H2¡¯, J = 8.4 Hz); 6.78 (d, 2 H, H3¡¯, J = 8.4 Hz); 5.85 (s, 2 H, C4-H); 4.46 (s, 4 H, N-CH2-pz); 3.69 (s, 6 H, N-CH3); 2.19 (s, 3 H, CH3-C6H4 ); 2.16 (s, 6 H, C5-CH3).
13C-NMR (CDCl3, 75 MHz): d= 149.72 (C3); 143.50 (C1¡¯); 139.51(C5); 129.85 (C3¡¯); 125.42 (C4¡¯); 113.54 (C2¡¯); 104.51(C4); 49.34 (N-CH2-pz); 36.25 (N-CH3); 20.62 (CH3-C6H4); 11.65 (C5-CH3).
MS (EI), m/z: 323 (5.4 %); 228 (0.4 %); 214 (100 %); 109 (24.5 %); 91 (4.4 %); 77 (0.5 %); 56 (4.9 %); 40 (3.5 %).
References and Notes:
1. Bouabdallah,
2. Bouabdallah,
3. Malek, F. Th¨¨se
de Doctorat d¡¯Etat, Facult¨¦ des Sciences,
Sample Availability: Available from MDPI.
© 2006 MDPI. All rights reserved.