|
Molbank 2006, M485 |
Synthesis of 6-chloro-2-oxo-1,2-dihydroquinoline-4-carbohydrazide
R. Bouhfid, E.M. Essassi*
Laboratoire de Chimie
Organique H¨¦t¨¦rocyclique, Universit¨¦ Mohammed V-Agdal, BP:
1014 Avenue Ibn Batouta,
E-mail :
[email protected]
*Author to whom correspondence
should be addressed
Received: 10 February 2006 / Accepted: 20 March 2006 / Published: 1 September 2006
Keywords: quinoline, hydrazide, condensation.
In previous
works we have shown that hydrazides are highly useful
starting materials and intermediates in the synthesis of several heterocyclic
compounds1-3 of potential biological activities.
The aim of
this work is to describe the preparation of a novel compound entitled
6-chloro-2-oxo-1,2-dihydroquinoline-4-carbohydrazide.
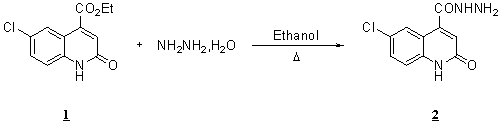
To a
solution of ethyl 2-oxo-1,2-dihydroquinoline-4-carboxylate 1 (1g,
3.9 mmol) in ethanol, was added hydrazine hydrate 80
% (0.22 mL, 4.6 mmol). The
mixture was refluxed for 24h and ice-water was added. The precipate
was filtered and recrystallised from ethanol to
afford
Melting point: >
¹H-NMR (300
MHz, DMSO): ¦Ä= 4.67 (NH2);
6.54 (s, 1H, =CH); 7.35-7.78 (m, 3H, HAr);
9.93 (NH).
¹³C-NMR (300 MHz, DMSO): ¦Ä= 121.8 (=CH); 118.0, 125.4, 131.2 (CHAr);
114.8, 126.3, 139.3, 139.9 (Cq); 161.3 (C=O); 165.0
(CON2H3).
MS (EI, m/z):
237.
Elemental analysis: Calculated for C10H8ClN3O2: C, 50.54 %; H, 3.39 %; N, 17.68 %; Found: C, 50.60 %; H, 3.42 %; N, 17.74 %;
Reference
1- Essassi EM., Fifani J., Bull. Soc. Chem.
Belg. 1987, 96, 63.
2- El Otmani B., El Mahi M., Essassi E.M., C. R. Chimie, 2002, 5, 517.
3- El Otmani B., El Hakmaoui A., Fifani J.,Essassi E.M.,Gueiffier A., C. R. Acad. Sci. Paris t-2, s¨¦rie IIc, 2001, 4, 285.
© 2006 MDPI. All rights reserved.