|
Molbank 2006, M486 |
Synthesis of 6-chloro-2-(propargyloxy)quinoline-4-carboxylic acid and propargyl
6-chloro-2-(propargyloxy)quinoline-4-carboxylate
R. Bouhfid, E.M. Essassi*
Laboratoire de Chimie
Organique H¨¦t¨¦rocyclique, Universit¨¦ Mohammed V-Agdal, BP:
1014 Avenue Ibn Batouta,
E-mail: [email protected]
*Author to whom correspondence should be addressed
Received: 10 February
2006 / Accepted: 20 March 2006 / Published:
1 September 2006
Keywords: quinoline, alkylation, esterification, propargyl bromide.
The
quinoline ring systems are important structural units
in naturally occurring alkaloids and synthetic analogues with interesting
biological activities. Therefore, the development of new and efficient
synthetic route for the preparation of their analogues is of importance in both
synthetic organic chemistry and medicinal chemistry.1-4
We reported here the synthesis of a new quinoline derivative.
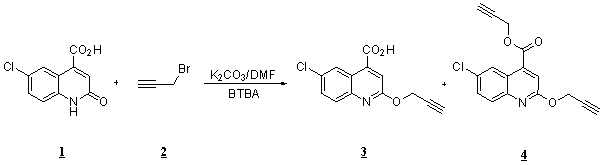
To a
solution of quinoline 1 (
6-chloro-2-(propargyloxy)quinoline-4-carboxylic acid, 3
Melting point: 170 ¡ãC
¹H-NMR (300
MHz, CDCl3): ¦Ä= 2.62 (t, 1H, ¡ÔCH, 3J= 2.4 Hz); 5.09 (d, 2H, OCH2, 3J=
2.4 Hz); 7.61 (s, 1H, =CH); 7.64-8.53 (m, 3H, HAr).
¹³C-NMR
(300 MHz, CDCl3): ¦Ä= 54.7 (OCH2); 76.1 (¡ÔCH); 118.1 (=CH); 127.1, 129.6, 133.7 (CHAr);
120.1, 137.3, 143.8 (Cq); 164.0 (C=N); 164.2 (CO2H).
MS (EI,
m/z): 237.
Elemental analysis: Calculated for C13H8ClNO3: C, 59.67 %; H, 3.08 %; N, 5.35 %; Found: C, 59.70 %; H, 3.04 %; N, 5.41 %;
Propargyl-6-chloro-2-(propargyloxy)quinoline-4-carboxylate,
4
Melting
point: 156 ¡ãC
¹H-NMR (300
MHz, CDCl3): ¦Ä=
2.67 (t, 1H, ¡ÔCH, 3J= 2.4 Hz); 2.70 (t, 1H, ¡ÔCH, 3J= 2.4
Hz); 5.05 (d, 2H, OCH2, 3J= 2.4 Hz); 5.12 (d, 2H, OCH2,
3J= 2.4 Hz); 7.08 (s, 1H, =CH); 7.58-8.20 (m, 3H, HAr).
¹³C-NMR
(300 MHz, CDCl3): ¦Ä= 52.4 (OCH2); 54.4 (OCH2); 78.1 (¡ÔCH); 78.7
(¡ÔCH); 78.9, 75.5 (¡ÔC); 118.2 (=CH); 125.5, 126.4, 131.9 (CHAr);
118.5, 138.1, 138.2 (Cq); 159.7 (C=N); 164.3 (CO2H).
MS (EI,
m/z): 299.
Elemental analysis: Calculated for C16H10ClNO3: C, 64.12 %; H, 3.36 %; N, 4.67 %; Found: C, 64.17 %; H, 3.29 %; N, 4.72 %;
References:
1.
Balasubramanian, M.;Keay, J. G. Pyridines and their Benzo Derivatives:
Application In Comprehensive Heterocyclic Chemistry II;Katrizky, A. P., Rees,
V. W., Scriven, E. F., Eds.;Pergamon: Oxford, 1996;Vol. 5, pp 245¨C300.
2. Ranu, B.
C.; Hajra, A.; Dey, S. S.; Jana, U. Tetrahedron 2003, 59, 813¨C819.
3. Wada, Y.; Mori, T.; Ichikawa, J. Chem. Lett. 2003,
32, 1000¨C1001.
4. Kobayashi, K.; Yoneda, K.; Mizumoto, T.; Umakoshi, H.; Morikawa, O.; Konishi, H. Tetrahedron Lett. 2003, 44,
4733¨C4736.
© 2006 MDPI. All rights reserved.