|
Molbank 2006, M488 |
Synthesis of
4-[4-(4-nitrobenzylideneiminophenylene)phenyleneimino methylidene] phenol
L. Marin*, V. Cozan
Phone:
0232-260332, Fax: 0232-211299, E-mail:
[email protected]
*Author to whom correspondence should be addressed;
Received: 26 October 2006 /
Accepted: 13 January 2006 / Published: 1 September 2006
Keywords: azomethine, liquid crystal, phenolic intermediate, thermotropic, nematic
The azomethine
1 was prepared as described elsewhere
[1]. The 4-[4-(4-nitrobenzylidene- iminophenylene)phenyleneimino methylidene] phenol (2) was obtained in a similar
manner [2] by reacting the azomethine 1 with
4-nitrobenzaldehyde in stoichiometric ratio:

A mixture of
Mp. 213 oC (by polarization light microscopy), 212 oC (by thermooptical analysis), 215 oC (by DSC method). In the melting state the product exhibit liquid crystalline behavior displaying a typical schlieren texture (see Figure 1). The isotropisation temperature could not be measured, because the decomposition started before (decomposition temperature To = 256oC)
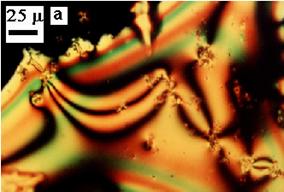
Figure 1. The schlieren texture of azomethine 2 at 246 oC,
between crossed polarizers
UV (DMF) 286, 382 nm
Anal. calc. for C26 H19 N3 O3 (421.45): N % 9.97; found: N % 9.52
IR (KBr, cm-1): 3450 (O-H), 1625 (CH=N), 1600, 1580
(C=C), 1520 (NO2 asym.), 1450 (C=C), 1350
(NO2 sym.),
1240 (C-O, asym.), 1160 (C-O, sym.), 855, 840
(1,4-phenylene ring).
References:
1. V.
Cozan, E. Avram Eur.
Polym. J. 2003, 39, 107-114.
2. C.
Racles, V. Cozan, High Performance Polymers 2002, 14, 169-181.
© 2006 MDPI. All rights reserved.