|
Molbank 2006, M489 |
Synthesis, Physical
Characterization, Antibacterial and Antifungal Activities of 2-((E)-1-(2-((E)-1-(2-Hydroxyphenyl)ethylideneamino) phenylamino) ethyl) phenol
A. A. Jarrahpour*1, A. F. Jalbout*2,
J. M. Brunel3, C. Loncle3,
1 Department
of Chemistry,
2 Department of Chemistry, The
3 Laboratoire SESNAB, Facult®¶ de St J®¶rôme,
Case 342, Universit®¶ Paul C®¶zanne, Av. Escadrille Normandie Ni®¶men, 13397 Marseille cedex 20,
France
Phone: +98 711 2284822,
Fax: +98 711 2280926
E-mail:
[email protected], [email protected]
*Author to whom correspondence
should be addressed
Received: 13
March 2006 / Accepted: 20 April 2006 / Published: 1 September 2006
Keywords: 2-Hydroxyacetophenone, 1,2-phenylenediamine,
Schiff base, AM1, B3LYP.
Abstract:
In this paper we report the synthesis of 2-((E)-1-(2-((E)-1-(2-hydroxyphenyethylideneamino)
phenylamino) ethyl) phenol.In addition to its
synthesis we present AM1 and B3LYP/6-31G* calculations to characterize the
physical properties of this molecule. Finally, the antifungal and
antibacterial activities of this derivative have been evaluated
Introduction
Schiff bases are an important class of ligands,
such ligands and their metal complexes have a variety
of applications including biological, clinical, analytical and industrial in
addition to their important roles in catalysis and organic synthesis [1].Those
that having multidentate coordination sites are known
to form complexes with transition metal ions readily [2].Such complexes play an
important role in bioinorganic chemistry and redox
enzyme systems [3] and may provide the basis of models for active sites of
biological systems [4] or act as catalysts [5]. Schiff base compounds are of
increasing interest because such dinuclear systems
are known to act as a paramagnetic building block for multidimensional expanded
structures as well as for their important roles in biological systems, e.g., in
many metalloenzymes, redox
and nonredox proteins and also as a catalyst in
olefin epoxidation [6]. Ardakani
and his coworkers have reported a selective nitrate PVC membrane electrode from
2-hydroxyacetophenone [7]. Synthesis and metal ion uptake studies of chelating
resins by use of 2-hydroxyacetophenone have been reported by Samal [8].

Results and Discussion:
1, 2- pheneylenediamine 1 (1.08g, 10mmol) and 2-Hydroxyacetophenone 2
(2.71 g, 20 mmol) were dissolved in 25 ml of warm
ethanol. The reaction mixture was refluxed for 7h at 85 °„C, and allowed to
stand. The solid crystals were filtered off and washed with ethanol. The pure
Schiff base 3 was isolated as a light brown crystalline solid (yield
89%).We
next performed theoretical calculations to present a viable structure for the
product. All calculations in this work where carried out with the AM1 level of
theory using the GAUSSIAN 03 suite of programs. More information about these
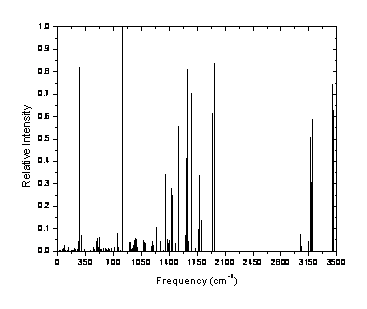
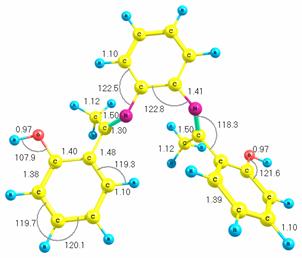
methods is available elsewhere. Figure 1 presents the optimized structure of
the molecule with bond lengths and bond angles shown. We obtained a melting
point (mp) value 108-110 °„C, and IR (KBr, cm-1):
3368(OH) (B3LYP/6-31G*: 3217); 1620(C=N) (B3LYP/6-31G*: 1629), as well as NMR.
1H-NMR: 14.27 (
13C-NMR: 173.12 (C = N), 162.13 (COH), 115.75-138.21 (aromatic carbons),
17.19 (CH3).
MS (m/z): 345 (M+1), 329, 227, 133, 65.
All calculations in this work where carried out with the AM1 level of
theory using the GAUSSIAN 03 [9] suite of programs. In addition we have carried
very intense B3LYP/6-31G* optimizations and frequency calculations. More
information about these methods is available elsewhere [10]. Figure 1 presents
the optimized structure of the molecule with bond lengths and bond angles shown
as well as the theoretical IR vibrational spectrum.
Table 1 shows the thermodynamic properties for the complex in figure 1
where T (temperature in K), S (entropy in J mol-1 K-1), Cp (heat capacity at
constant pressure in kJ mol-1 K-1), and ¶§H=H°„ - H°„298.15 (enthalpy content, in
kJ mol-1), T1=100 K, T2=298.15 K, and T3=1000 K calculated AM1 and B3LYP/6-31G*
frequencies. The fits were performed according to the equations implemented by
the National Institute of Standards and Technology (NIST) [11]. These equations
have been very good at predicting physical properties of various molecules, as
we have tested in the past [12-14].
3
Figure
1. AM1 optimized
structure and its theoretical IR vibrational spectrum
for molecule 3.
|
|
|
Fitted Thermodynamic Equation (T/1000=t) |
100 K |
298.15 K |
1000 K |
|
3 |
Cp |
-43.40526+ 1651.94082*t -933.47093*t2 + 174.57639*t3 +0.49907*t-2 |
162.2 |
373.91 |
851.29 |
|
S |
42.38515*ln(t) + 1150.64098*t +
29.96975*t2/2 -367.20075*t3/3 + 723.17337/(2*t2)
+ 162.84889 |
432.48 |
703.25 |
1444.07 |
|
|
¶§H |
133.5428*t +1417.88899*t2/2
-523.88367*t3/3 -52.55339*t4/4 +0.2264 /t -563.55851 |
10.32 |
63.16 |
526.8 |
|
|
|
|
|
|
|
Table 1. Thermodynamic properties of the molecules in Figure 1-2 (A-B), calculated at the AM1 level and B3LYP/6-31G* level of theory, where Cp is the heat capacity in J mol-1 K-1, S is the entropy in J mol-1 K-1, and ¶§H is the standard enthalpy kJ mol-1. These where fitted to the Shomate equations which are implemented by the JANAF tables of the NIST databases. These equations converged to an R2 value of 0.999 on average.
Antibacterial and antifungal activity tests
Derivative 3
was evaluated for its in
vitro biological properties against human pathogens [15]. This compound was found to
possess no antifungal activities against S. cerevisiae
(ATCC 28383) and no antibacterial activities against
Gram-positive and Gram-negative bacteria have been noticed (Table 2).
|
Sample CIP |
Antimicrobial
activity (MIC), µg/mL |
|||
|
S. cerevisiae (ATCC 28383) |
S. aureus (4.83) |
C. albicans (1180-79) |
E. Coli (54127) |
|
|
3 |
>50 |
>50 |
>50 |
>50 |
Table 2. Antimicrobial activity of Schiff base 3
References:
[1]. Zalp-Yaman,
S. E; Kasumov, V. T; Ahmet, M. O. Polyhedron 2005, 24,
1821.
[2]. Samal, S.; Mohapatra, N. K.; Acharya, S.; Dey, R. K. Reac. & Func. Polym. 1999, 42, 37.
[3]. KolodZiej,
A. F. Prog. Inorg. Chem. 1994, 41, 493.
[4]. Bouwman,
E; Henderson, R. K; Reedijk, J; Veldman, N; Spek, A. L. Inorg. Chim. Acta 1999, 278, 105.
[5].
Meseguer, M; Moreno-Man, M; Vallribera, A. Tetrahedron Lett. 2000,
41, 4093.
[6].
Karmakar, R.; Choudhury, C. R.; Bravic, G.; Sutter, J. P.; Mitra, S. Polyhedron
2004, 23, 949.
[7].
Mazloum Ardakani, M.; Salavati-Niasari, M.; Jamshidpoor, M. Sensors and
Actuators 2004, 101, 102.
[8]. Samal, S;
Acharya, S; Dey, R. K; Ray, A. R. Talanta 2002, 57, 1075.
[9]. Frisch, M.J., et.al., GAUSSIAN 03, Revision A.1, M. J. Frisch, et. Al.,
Gaussian, Inc.,
[10]. Foresman, J.B., Æ Frisch, ,
Exploring Chemistry with Electronic Structure Methods, 2nd edition Gaussian,
INC,
[11]. Linstrom, P.J., Mallard, W.G., Eds., NIST Chemistry WebBook, NIST Standard Reference
Database Number 69, July 2001, National Institute of Standards and Technology,
Gaithersburg MD,
20899 (http://webbook.nist.gov).
[12]. Jalbout, A.F. , Solimannejad, M., Labonowski,
J.K., Chem. Phys. Letts. 2003,
379, 503.
[13]. Jalbout, A.F., Jiang, Z.-Y., Quasri,
A., Jeghnou, H., Rhandour,
A., Dhamelincourt, M.C.,
Dhamelincourt, P., Mazzah,
A., Vib. Spect. 2003, 33, 21.
[14]. Jalbout, A.F., Nazara, F., Turker, L., J. Mol. Struct.
(THEOCHEM), 2004, 627, 1. (Invited Review)
[15]. Growth was measured in
vitro using a liquid-phase method according to NCCLS guidelines
from the American Society of Microbiology for 24 hours using various concentrations of drugs.Dei Cas, E.; Dujardin, L.; Ribeiro Pinto, M.
E.; Ajana, F.; Fruit, J.; Poulain, D.; Camus, D.; Vernes, A.; Francois, N. Mycoses 1991, 34, 167-172
© 2006 MDPI. All rights reserved.