|
Molbank 2006, M491 |
New 1-(ethyl-ethanoate-yl)-5,5¡¯-diisopropyl-3,3¡¯-bipyrazole
Ibrahim Bouabdallah,1*
Ismail Zidane,1 Rachid
Touzani,1 Abdelkrim Ramdani,1 Abraham
F. Jalbout2 and B. Trzaskowski2
1Laboratoire de Chimie Organique Physique, D¨¦partement de Chimie, Facult¨¦ des Sciences, Universit¨¦ Mohamed Premier, BP 524, 60000, Oujda, Maroc.
1NASA
e-mail : [email protected]
*Author to whom correspondence should be addressed
Received: 1 May 2006 / Accepted:
18 July 2006 / Published: 1 September 2006
Keywords: Bipyrazol, bidentate ligand and aryl group.
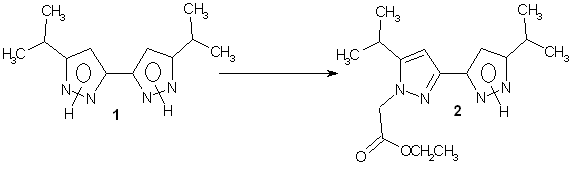
To a
solution of 5,5¡¯-diisopropyl-3,3¡¯-bipyrazole 1 [1] (218 mg, 1 mmol) in DMSO (1 ml) was added solid potassium tert-butoxide (112 mg, 1 mmol)
followed by addition of ethyle chloroacetate
(123 mg, 1 mmol) in DMSO (1 ml) through a syringe.
The resulting mixture was heated to
Yield: (60 mg, 20 %).
Melting point: 124 - 125¡ãC.
IR (KBr, cm-1): 3220 (¦ÍN-H) ; 2930 ; 2865 (¦ÍC-H, CH3) ; 2725 ; 2620 (¦ÍC-H, CH(CH3)2) ; 1670 (¦ÍC=O) ; 1545 (¦ÍC=N) ; 1495 (¦ÍC=C) ; 1430 ; 1365 ; 1310 ; 1295; 1180 ; 1170 (¦ÍC-O-C) ; 1165 ; 1110 ; 1075 ; 1020 ; 1005 ; 923; 780 ; 776 ; 695.
1H-NMR (CDCl3, 300 MHz ): ¦Ä= 6.36 (s, 2 H,C4-H) ; 4.87 (s, 2 H, N-CH2-CO2Et) ; 4.20 (q, 2 H, O-CH2-CH3, J = 6.9 Hz) ; 3.01 (sept, 1 H, CH(CH3)2, J = 6.9 Hz) ; 2.78 (sept, 1 H, CH(CH3)2, J = 6.9 Hz) ; 1.24 (m, 15 H, CH(CH3)2, CH2-CH3).
13C-NMR (CDCl3, 75MHz
): ¦Ä= 31 (C=O);
151.91 (C3); 146.36 (C5); 100.61 (C4); 99.92 (C4); 62.20 (N-CH2-CO2Et);
51.00 (O-CH2CH3); 27.19 (CH(CH3)2);
25.93 CH(CH3)2) ; 23.07 (CH(CH3)2)
; 22.95 (CH(CH3)2) ; 14.49 (CH2-CH3).
MS (EI), m/z: 304; 289; 276; 231; 217; 189; 167; 149; 120; 94; 83; 71; 57; 43.
Elemental Analysis: Calculated for C16H24N4O2:
C 63.15, H 7.89, N 18.42, Found: C 63.08, H 7.90, N 18.39.
In addition
to the experiments we did theoretical calculations. All calculations in this work where carried out with the AM1 level of
theory using the GAUSSIAN 03 [2] suite of programs. More information about these methods is available
elsewhere [3-4].
Table 1 shows the thermodynamic
Parameters for the product where T (temperature in K), S (entropy in J mol-1
K-1), Cp (heat capacity at constant pressure in kJ mol-1
K-1), and ¦¤H=H¡ã - H¡ã298.15 (enthalpy content, in kJ mol-1),
T1=100 K, T2=298.15 K, and T3=1000 K
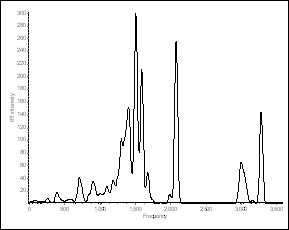
calculated AM1 frequencies. The theoretical vibrational
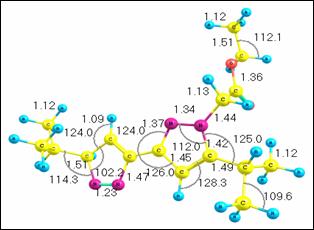
spectrum and structure are shown above as well as the structure is also shown
in the table. In the structure, all bond lengths are in angstroms (Å) and bond
angles are in degrees (¡ã) and the frequencies are in cm-1, and the
IR intensities in KM/mol (broadened by the Doppler method). These calculations
are useful for future thermodynamic studies as well as for NIST database
indexing. The high values for this molecule suggests higher thermodynamic
stability for this complex, also justifying the fact that it should be
observed, even at high temperatures.
We also compute a hydration energy of ¨C0.27 kcal/mol, Log P of 0.90
(Clog P=1.8694) and a polar surface area of 77.5, which correlates well to the
hydration energy (both rather low).
|
|
100 K |
298.15 K |
1000 K |
1200 K |
1500 K |
2000 K |
|
Cp |
177.68 |
356.34 |
832.63 |
895.22 |
960.29 |
1023.77 |
|
S |
451.80 |
725.30 |
1437.77 |
1595.40 |
1802.68 |
2088.61 |
|
¦¤H |
11.33 |
64.03 |
511.35 |
684.46 |
963.49 |
1461.21 |
Table
1. Physical properties, thermodynamic equations, as well as structural
AM1 geometries.
References:
1. Bouabdallah, I.; Ramdani, A.; Zidane,
2. Frisch, M. J., et. Al., GAUSSIAN 03, Revision A.1,
Gaussian, Inc.,
3. Foresman, J.B., Frisch, Æ, Exploring Chemistry with Electronic
Structure Methods, 2nd edition Gaussian, INC, Pittsburgh, PA, 1996
4. Jalbout, A.F., Nazari, F., Turker, L., J. Mol. Struct.
(THEOCHEM), 1, 2004, 627 ; Jalbout, A.F., Adamowicz, L., Adv.
.Quant. Chem.(Book Series, Ed. J. Sabin)., 2006,
xxx-xxx (Reviews
© 2006 MDPI. All rights reserved.