|
Molbank 2006, M499 |
(4Z)-1-Benzyl (1,3-Dibenzyl)-4-(2-oxopropylidene)-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one
Ould Mohamed Sidya Mohamed Said,1
Bouhfid Rachid,1 Nicolas Joly,2
Vincent Lequart,2 Patrick Martin,2 M. Massoui1
and El Mokhtar Essassi1*
1 Laboratoire de Chimie Organique H®¶t®¶rocyclique ,
Facult®¶ des Sciences, Avenue Ibn-Batouta,
2 Blood-Brain
Barrier Laboratory (EA 2465), IUT of B®¶thune,
E-mail : [email protected]
*Author to whom correspondence should be addressed
Received: 18 July 2006 / Accepted: 20 July 2006 /
Published: 1 September 2006
Keywords: alkylation, benzodiazepinones, benzylchlorid.
We describe
in this work the synthesis of new benzodiazepine derivatives susceptible to
possess various pharmacological activities.
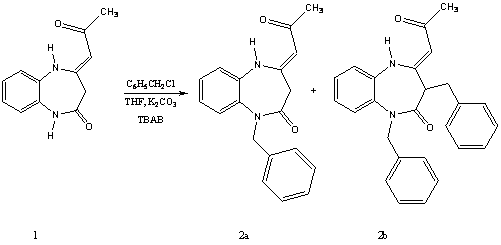
To a solution of 4Z-2-oxopropylidene)-1,3,4,5-tetrahdro-2H-1,5-benzodiazepin®C2-one 1[1,2] in tetrahydrofuran (60mL), was added K2CO3 (0,02 mole, 2,76 gm), benzylchlorid (0,02 mole, 2,52 gm) and n-tetrabutylammonium bromid (0.001 mole, 0,321 gm). The mixture was stirred at room temperature for 48 hours. The soultion was filtered by suction filtration. The solvent was removed under reduced pressure. The residu was chromatographed on silica gel column using hexane and ethyl acetate (80/20) as eluent to afford the products 2a and 2b as white solids.
(4Z)-1-benzyl-4-oxopropylidene)-1,3,4,5-tetrahdro-2H-1,5-benzodiazepin®C2-one,
2a
This
compound was obtained in 55% yield;
Melting Point: 165-167 °„C
MS(IE):M.+ m/z=306
1H-NMR (250 MHz, CDCl3): ¶ń= 2.1
(s,3H,CH3), 3.13(HAHB,, 2J=15.47
Hz, ![]() ), 5.0 (s,2H,
), 5.0 (s,2H, ![]() ), 5.3 (s,1H,
), 5.3 (s,1H, ![]() ),7.1 -7.3(m, 4H, HArm),
12.2(s,1H,NH).
),7.1 -7.3(m, 4H, HArm),
12.2(s,1H,NH).
13C-NMR (62.9 MHz, CDCl3): ¶ń= 29,.5,
41.4, 52.2 , 96.5, 123.2, 123.4, 125.5, 126.6, 126.7,
127.3, 128.7, 132.9, 134.7, 155.6, 167.2, 198.2.
Elemental analysis : Calculated for C19H18N2O2 : C, 74.49 %; H, 5.92 %; N, 9.14 %; Found: C, 74.52 %; H, 5.85 %; N, 9.22 %;
(4Z)-1,3-dibenzyl-4-oxopropylidene)-1,3,4,5-tetrahdro-2H-1,5-benzodiazepin®C2-one,
2b
This
compound was obtained in 20% yield;
Melting Point: 145-147 °„C
1H-NMR (250 MHz, CDCl3): ¶ń= 2.2 (s,3H,CH3), 2.9(m,3H
,CH-CH2 ), 4.8(HAHB,,
2J=15.9Hz, ![]() ), 5,4(s,1H,
=CH), 7.1-7.3(m, 4H, HArom),
12.6(s, 1H, NH)
), 5,4(s,1H,
=CH), 7.1-7.3(m, 4H, HArom),
12.6(s, 1H, NH)
13C-NMR (62.9 MHz, CDCl3): ¶ń= 29.9, 31.8, 47.4, 52.7, 93 122.8,
123.2, 125.6, 126.2, 126.5, 126.6, 127.1, 128.4, 128.6, 129.3, 132.0, 135.0,
136.8, 138.7, 158.5, 167.3, 198.0.
Elemental analysis: Calculated for C26H24N2O2: C, 78.76 %; H, 6.10 %; N, 7.07 %; Found: C, 78.80 %; H, 6.04 %; N, 7.10 %;
References
1. El Abbassi M., Essassi E.M. and Fifani J.; Tetrahedron Lett, 1987, 28, 1389.
2. Djerrari B., El Abbassi M., Essassi E.M. and Fifani J.; Tetrahedron Lett., 1989, 30, 7069.
© 2006 MDPI. All rights reserved.