|
Molbank 2006, M509 |
Synthesis of N, N-Dimethyl-3-phenoxyquinoxalin-2-amine
Craig A. Obafemi*1,
Wolfgang Pfleiderer2
1Department of Chemistry,
2Fachbereich Chemie,
Konstanz University D-78457 Konstanz/ Germany
E-mail:
[email protected]
([email protected])
*Author to whom
correspondence should be addressed
Received: 7
February 2005 / Accepted: 2 February 2006 / Published: 1 December 2006
Keywords: Substitution reaction, 2,3-dichloroquinoxaline, phenol.
The two chloro groups in 2,3-dichloroquinoxaline 1 can be displaced by nucleophiles, a process that may take place in a stepwise manner [1]. 1 was reacted with phenol 2 in dimethylformamide (DMF) to give N,N-dimethyl-3-phenoxyquinoxalin-2-amine 3.
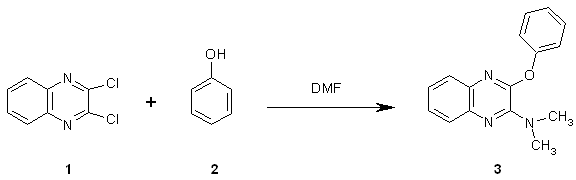
A mixture of 2,3-dichloroquinoxaline 1 (5.0 g, 25 mmol), phenol (1.2 g, 13 mmol) and Na2CO3 (0.7 g, 7 mmol) in DMF (40 mL) was heated to reflux for 10 h. with magnetic stirring. The reaction mixture was cooled and poured into water (200 mL) to give a solid product. Flash vacuum column chromatography (silica gel, petroleum ether (b.p. 100¡ã)/EtOH 100:1) gave pure N,N-dimethyl-3-phenoxyquinoxalin-2-amine 3 (2.1 g, 62%, based on phenol).
Melting point: 85 ¨C 86¡ãC.
IR (nmax, KBr, cm-1): 2940, 2880 (C-H), 1578 (C=C), 1516, 1196.
1H-NMR (400 MHz, CDCl3, d (ppm): 7.72 (d, 1H, J = 8.47 Hz, Ar-H), 7.52 (d, 1H, J = 9.25 Hz, Ar-H), 7.47 ¨C 7.34 (m, 3H, Ar-H), 7.31 ¨C 7.21 (m, 4H, Ar-H), 3.31 (s, 6H, 2 x CH3).
13C-NMR (100 MHz, CDCl, d (ppm): 152.9, 149.6, 147.7, 139.5, 135.4, 129.5, 127.3, 126.6, 125.7, 125.0, 124.9, 121.6, 40.6.
Acknowledgment
We thank the Alexander von Humboldt Foundation for a post-doctoral fellowship (CAO).
Reference
1.
© 2006 MDPI. All rights reserved.