|
Molbank 2006, M511 |
4,4'-Diethylaminoethoxyhexestrol dihydrochloride
Behrooz
H. Yousefi and Ulrich Jordis*
Institute of Applied Synthetic
Chemistry,
E-mail: [email protected]
*Author to whom correspondence should
be addressed
Received: 23 November 2004 / Accepted: 10 November
2006 / Published: 1 December 2006
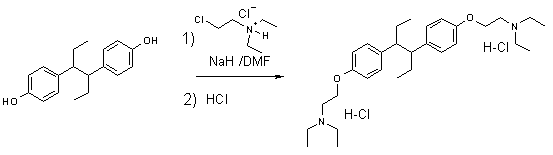
The bis-diethylaminoethylether of hexestrol
has been shown to be hypocholesteremic by inhibiting the reduction of
desmosterol to cholesterol.1 The hypocholesteremic activity of the
title compound in man has been reported2 and its mode of action
confirmed.3
The histopatological features of non-alcoholic steatohepatitis were described
in patients taking 4,4¡¯-diethylaminoethoxyhexestrol.4,5 In the
context of a joint collaboration in a genomics project, we aimed to prepare
the title compound in multi-grams scale. We report here new preparation with
significant yield improvement of title compound. For this purpose the preparation
of 4,4'-diethylaminoethoxyhexestrol dihydrochloride under different reported
and analogue conditions have been tried. According to the Hughes et al.
method,1 the reaction using sodium ethoxide in toluene gave 46% yield, and there is
no yield improvement using sodium methoxide in ethanol and toluene.6
The preparation with K2CO3 in acetone7 or DMF8
gave similar results. We prepared the title compound by treating hexestrol with
NaH in DMF to afford the sodium phenolate which was reacted with
diethylaminoethylchloride hydrochloride followed by treatment with ethereal HCl
in 85% overall yield, which is similar to the Ezquerra et al. method in preparation
of [2-(3-Benzyl-3H-benzoimidazol-5-yloxy)-ethyl]-dimethyl-amine.9
Experimental: To a suspension
of NaH (9 g, ~50% in mineral oil, ~190 mmol) in dry DMF (250 mL), hexestrol
(13.6 g, 50 mmol) was added and the mixture heated up to 90ºC for 30 min. under
argon. Then a solution of diethylaminoethylchloride hydrochloride (20 g, 116
mmol) in dry DMF (100 mL) was added drop wise and the reaction followed by TLC.
After 2 hours no starting material was detectable. Water (20 mL) was added to
the reaction mixture and all volatiles evaporated in vacuo. The residue was extracted using diethyl ether (3¡Á200 mL)
and the combined organic phase washed with water and brine, dried over MgSO4,
filtered and the solvent evaporated in vacuo.2 The resulting oil was
dissolved in dry ethanol and the solution added drop wise to cold dry diethyl
ether saturated with HCl (500 mL) and kept in the refrigerator overnight to get
crude crystalline 4,4'-diethylaminoethoxyhexestrol dihydrochloride (21.8 g).
The product was re-crystallized from ethyl acetate and ethanol to yield a first
fraction of 19.6 g (72%, >99% HPLC purity). Further workup of the mother
liquor resulted in a total product of 85%.
Melting
Point: 225.5¡ãC
(Lit. 223-226¡ãC),
Elemental
Analysis: Calculated for C30H48N2O2
.2 HCl: C, 66.53%, H, 9.30%, N, 5.17%, Cl, 13.09%; Found: C, 65.55, H, 9.64, N,
5.07, Cl, 12.61.
1H NMR (DMSO-d6) d 0.38 (t, 6H),
1.24 (t, 16H), 2.54 (b, 2H), 3.21 (q, 8H), 3.45 (b, 4H), 4.28 (b, 4H), 7.09
(dd, 8H).
13C NMR (DMSO-d6) d 8.2, 11.8, 26.8,
47.4, 50.1, 52.3, 61.8, 65.0, 114.3, 129.1, 137.2, 155.4.
References:
1.
Hughes, G. M. K.; Moore, P. F.; Stebbins, R. B. J.
Med. Chem. 1964, 7, 511-18.
2.
Zimmerman, H. Drugs 1978, 16,
25¨C45.
3.
Annoni, G.; Longaretti, A. Med. Welt 1961,
1945-1947.
4.
Phillips, Wm. A.; Avigan J. Proceedings of the
Society for Experimental Biology and Medicine 1963, 112, 233-6.
5.
a) Stremmel, W.; Blechacz, B.; Herrmann, T.; Rost,
D.; Mueller, S. Der
Internist 2001, 42, 1641¨C1650. b) Oneta, C.
M.; Dufour, J. F. Swiss Med. Wkly. 2002, 132, 493¨C505.
6.
El-Wakil,
H.; Seitz, D. E.; Mansour, E. S. M. E. Heteroatom Chem. 1991, 2,
583-586.
7. a)
Mehta,L. K.; Parrick, J. J. Heterocycl. Chem. 1995, 32,
391-394. b) Reddy, Y. T.; Reddy, P. N.; Rao, M. K.; Rajitha, B.;
Reddy, S. M. Indian J. Chem. Sect. B Org. Chem. Inc.l Med. Chem. 2001,
40, 479-483.
8.
Olier-Reuchet,
C.; Aitken, D. J.; Bucourt, R.; Husson, H. P. Tetrahedron Lett. 1995,
36, 8221-8224.
9. Ezquerra, J.; Lamas, C.;
Pastor, A.; Garcia-Navio, J. L.; Vaquero, J. J. Tetrahedron 1997,
53, 12755-12764.
10. An analytical
sample of the free base was obtained by flash chromatography on silica (1:1,
petrol /ethyl acetate). Mp:
47¡ãC and the structure confirmed by NMR: 1H
NMR (CDCl3) d 0.45 (t, 6H), 1.072 (t, 12H),
1.24 (m, 4H), 2.42 (m, 2H), 2.59 (q, 8H), 2.85 (t, 4H), 3.97 (t, 4H), 6.89 (dd,
8H).
© 2006 MDPI. All rights reserved.