|
Molbank 2006, M512 |
Spectroscopic
and ab-initio study of schiff
base ligand 4-[(2-hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol-3-one
Kiran R. Suratia*, B. T. Thakerb and Gavrang Shahb
aShri
bDepartment of Chemistry,
e-mail: [email protected]
*Author to whom correspondence should be addressed
Received:
Key
words: Pyrazolone Schiff base, 3D-modeling, tautomerism,
Molecular structure
Introduction
Pyrazolone ring systems represent an important class of compounds [1] not only for their theoretical interest but also for their number of application in diverse area [2-6]. 1-Phenyl pyrazolone derivatives have interesting pharmacological properties as analgesic [7] antipyretic [8] and antiinflamatory agents [9]. The classes of chelating extractants which have received the most attention in recent years have the basic structure of pyrazole. Due to the above reasons we have take interest to synthesize this class of ligands [10], and take interest of their spectroscopic as well as theoretical study. The electronic structure of the Schiff base ligand (III) was optimized using 6-311G basis set at HF level ab-initio studies. The attempt was made to predict the coordinating atoms of the ligand. The electron correlation at MP2 level was also performed.
Experiment Section
5-hydroxy-3-methyl-1-p-tolyl-1H-pyrazole-4-carbaldehyde(II)
was prepared by condensation of 5-methyl-2-p-tolyl-2H-pyrazol-3-ol
(I) with DMF and POCl3 [11]. 5-methyl-2-p-tolyl-2H-pyrazol-3-ol
(I) (9.4 g, 0.05 mol) in DMF (10 cm3, 0.05
mol) was cooled to 0 to 10∼C in an ice bath. To this phosphoryl chloride (5.5 cm3, 0.06
mol) was added dropwise at a rate to maintain
the temperature between 10 and 20∼C. After the addition was complete, the
reaction mixture was heated on a steam bath for 2.5 hours then poured into 1L
of ice water. The resulting mixture was allowed to stand overnight and product
was collected by filtration, washed with water, and dried.
4-[(2-Hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol-
3-one(III) Schiff base was synthesized by refluxing methenolic
solutions of ethanolamine (
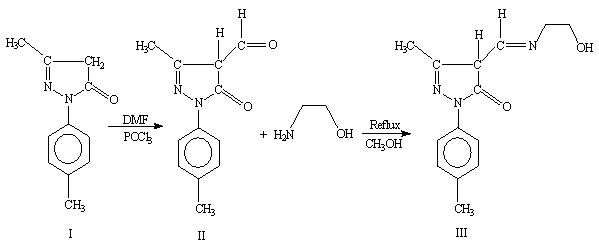
Results and
Discussion
Schiff base ligand 4-[(2-hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol-3-one (III) characterized by the spectroscopic data, as shown below. Schiff base ligand (III) shows tautomeric form imine-ol and amine-one, (Figure 1) these forms are active in solution and in solid state respectively [12]. Also the attempt to carried out the ab-initio study of this ligand, the full geometry optimization of schiff base ligand (III) has been carried out in cartesian coordinates by quasi-Newton-Raphson gradient method using the restricted Hartree-Fock (RHF) approximation with help of Gamess program package [13]. The present compound contains too many hetero atoms. Thus it is interesting to know that out of these which one are the coordinating atoms. The lowdin atomic charges on N (schiff base N) and O (enolic O of pyrazoline) are -0.243106 and -0.435565, respectively, which are highest negative charges among all hetero atoms suggesting that these atoms are coordinating to metals. The lowdin charge on O (O of ethanolamine) is -0.416095 which is comparable with O but the stereochemistry of this oxygen is such that it is away from the bite site and hence coordination through this oxygen is not possible. The bond-stick and space filling diagram of schiff base ligand shown in figure 2 and 3 respectively.
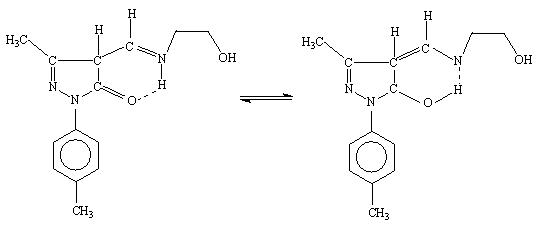
Figure 1. Tautomerism between imine-ol
and amine-one form
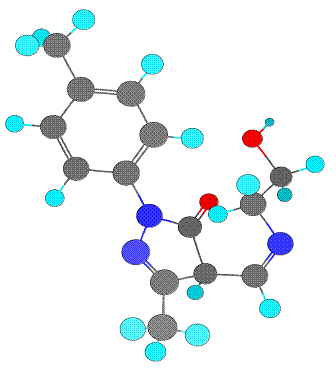
Figure 2. Ball and stick model schiff base ligand (III)
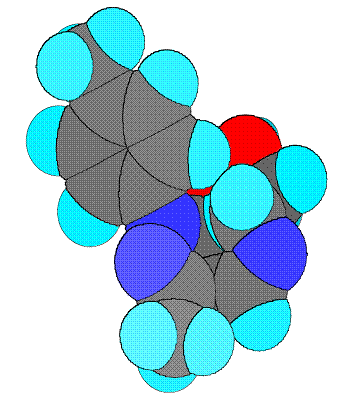
Figure 3. Space filling diagram of schiff base ligand(III)
5-Hydroxy-3-methyl-1-p-tolyl-1H-pyrazole-4-carbaldehyde
(II)
Yellow crystal
obtained, yield 76%
Melting point:
184 ∼C
FT-IR (KBr) umax(cm-1): 3400-3300m (OHhydrogen
bonded), 2809-2728vs (C每Haldehydic
, doublet), 1674s (C=O), 1598vw (C=Ncyclic
).
1H-NMR
(300 MHz, CDCl3): 汛= 9.65 (s, 1H, CHO), 8.45 (s (enolic),
13C-NMR
(75 MHz, CDCl3): 汛= 17.4, 20.9 (CH3); 120.3, 129.4, 133.3, 137.8 (benzene ring) ; 60.8, 155.6,
(heterocyclic ring), 169.89 (C=O), 199.5 (H 每C=O) aldehyic
MS (LC-MS): (Calcd. for m/z [M]+ 216
) 217 [M+1]+
Elemental
analysis: Calculated for C12H12N2O2:
C 66.3; H 5.6; N 12.9; found: C 66.1; H 5.3; N 12.9%.
4-[(2-hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol-3-one
(III)
Brown crystal
obtained, yield 82%
Melting point:
157 ∼C
FT-IR (KBr) umax(cm-1): 3294m (O每H), 2946-2873m (C每H),
1646 (C=N), 1676s (C=O), 1598vs (C=Ncyclic).
1H-NMR (300 MHz, CDCl3): 汛= 9.73 (s, 1H, 每OHenol); 7.93 (s, 1H, 每HC=N) ; 7.87 (m, 2H J = 8.4, ortho- to pyrazoline ring); 7.34 (d, 2H J = 8.4, m- to pyrazoline ring); 5.12 (s, 1H, 每OH); 3.56 (t, 2H J = 4.8, NCH2); 3.48 (t, 2H J = 4.8, CH2O); 2.32 (s, 3H, CH3); 1.23 (s ,3H, CH3).
13C-NMR
(75 MHz, CDCl3): 汛= 17.3, 20.9 (CH3); 56.0, 64.2 (CH2); 120.3, 129.4,
133.3, 137.8 (benzene ring) ; 43.6, 155.6, (heterocyclic ring), 169.89 (C=O).
Elemental
analysis: Calculated for C14H17N3O2:
C 64.85; H 6.6; N 16.21 Found: C 64.9; H 6.3; N 16.2%.
MS
(LC-MS): (Calcd.
for m/z [M]+ 259.13) 260 [M+1]+
Acknowledgement
We express our sincere thanks to CDRI,
References
1. Elguero, J. in "Comprehensive Heterocyclic Chemistry ". Katritzky A. R.; Rees C. W.; Potts K. T.; Eds. Pergamon, Oxford, 1984, Vol. 5, p. 167.
2. Gibson, D., Coord .Chem. Rev. 1969, 4, 163.
3. Elmorsi, M. A.; Hassanein, A.M.; Corros. Sci. 1999, 41, 2337.
4. Ito, T.; Goto, C.; Noguchi, K.; Anal. Chim .Acta. 2001, 443, 41.
5. Yang, L.; Jin,
W.; Lin, J.; Polyhedron 2000, 19, 93.
6. Ito, T., J. Electroanal .Chem. 2001, 495, 87.
7. Brogden, R.
N., Drugs 32 60 (1986).
8. Stoltz, M ;
Oliver, D. W.; Wessels, P. L. ; Chalmers, A. A. J. Pharm. Sci., 1991,
80, 357.
9. Schillaci, D.;
Maggio, B.; Raffa, D.; G. Farmaco., 1992, 47, 127.
10. Surati, K.
R.; Thaker B. T.
J. Coord. Chem. 2006, 59,1191.
11. Wallace, D. J.; Straley, J. M..; J.Org. Chem. 1961, 26, 3825
12. Surati K. R.,
Ph. D. Thesis, Department of Chemistry, V.N.S.G.University,
13. Schmidt, M. W.; Baldridge, K. K.; Boatz J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S. ; Matsunaga, N.; Nguyen, K. A.; Su, S. J.; Windus, T. L.; Dupuis, M., Montgomery, J. A. J. Comput. Chem.1993, 14, 1347.
© 2006 MDPI. All rights reserved.